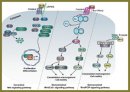
Wnt Signaling Pathway and Its Significance for Melanoma Development
Melanoma is characterized by its high metastatic propensity. Melanoma metastasis is associated with an activation of signaling pathways that are responsible for embryogenesis. Wnt signaling pathway is considered as one of the key signaling cascades, whose aberrant activation results in melanoma development. Wnt signaling includes a complex network of intracellular interactions. Its ligands are able to initiate at least three signal transduction pathways: canonical and two noncanonical. According to modern views, canonical branch of Wnt signaling pathway is involved in cell proliferation and differentiation. Noncanonical Wnt signaling pathways, on the contrary, control cytoskeleton organization and cell motility. Currently, canonical and noncanonical Wnt signaling cascades are supposed to affect different stages of tumor progression. Canonical Wnt signaling pathway contributes to melanoma formation, while noncanonical branches of Wnt signal transduction are involved in metastasis.
- Miller J.R. The Wnts. Genome Biol 2002; 3(1): 1–15.
- Smolich B.D., McMahon J.A., McMahon A.P., Papkoff J. Wnt family proteins are secreted and associated with the cell surface. Mol Biol Cell 1993; 4(12): 1267–1275.
- Willert K., Brown J.D., Danenberg E., Duncan A.W., Weissman I.L., Reya T., Yates J.R., Nusse R. Wnt proteins are lipid-modified and can act as stem cell growth factors. Nature 2003; 423(6938): 448–452.
- Slusarski D.C., Corces V.G., Moon R.T. Interaction of Wnt and a Frizzled homologue triggers G-protein-linked phosphatidylinositol signalling. Nature 1997; 390(6658): 410–413.
- Tamai K., Semenov M., Kato Y., Spokony R., Liu C., Katsuyama Y., Hess F., Saint-Jeannet J.P., He X. LDL-receptor-related proteins in Wnt signal transduction. Nature 2000; 407(6803): 530–535.
- Pinson K.I., Brennan J., Monkley S., Avery B.J., Skarnes W.C. An LDL-receptor-related protein mediates Wnt signalling in mice. Nature 2000; 407(6803): 535–538.
- Gordon M.D., Nusse R. Wnt signaling: multiple pathways, multiple receptors and multiple transcription factors. J Biol Chem 2006; 281(32): 22429–22433.
- Oishi I., Takeuchi S., Hashimoto R., Nagabukuro A., Ueda T., Liu Z.J., Hatta T., Akira S., Matsuda Y., Yamamura H., Otani H., Minami Y. Spatio-temporally regulated expression of receptor tyrosine kinases, mRor1, mRor2, during mouse development: implications in development and function of the nervous system. Genes Cells 1999; 4(1): 41–56.
- Hovens C.M., Stacker S.A., Andres A.C., Harpur A.G., Ziemiecki A., Wilks A.F. RYK, a receptor tyrosine kinase-related molecule with unusual kinase domain motifs. Proc Natl Acad Sci USA 1992; 89(24): 11818–11822.
- Lu W., Yamamoto V., Ortega B., Baltimore D. Mammalian Ryk is a Wnt coreceptor required for stimulation of neurite outgrowth. Cell 2004; 119(1): 97–108.
- Keeble T.R., Halford M.M., Seaman C., Kee N., Macheda M., Anderson R.B., Stacker S.A., Cooper H.M. The Wnt receptor Ryk is required for Wnt5a-mediated axon guidance on the contralateral side of the corpus callosum. J Neurosci 2006; 26(21): 5840–5848.
- Clevers H. Wnt/beta-catenin signaling in development and disease. Cell 2006; 127(3): 469–480.
- Weeraratna A.T. A Wnt-er wonderland--the complexity of Wnt signaling in melanoma. Cancer Metastasis Rev 2005; 24(2): 237–250.
- Bilic J., Huang Y.L., Davidson G., Zimmermann T., Cruciat C.M., Bienz M., Niehrs C. Wnt induces LRP6 signalosomes and promotes dishevelled-dependent LRP6 phosphorylation. Science 2007; 316(5831): 1619–1622.
- Kuhl M., Sheldahl L.C., Park M., Miller J.R., Moon R.T. The Wnt/Ca2+ pathway: a new vertebrate Wnt signaling pathway takes shape. Trends Genet 2000; 16(7): 279–283.
- Dissanayake S.K., Wade M., Johnson C.E., O’Connell M.P., Leotlela P.D., French A.D., Shah K.V., Hewitt K.J., Rosenthal D.T., Indig F.E., Jiang Y., Nickoloff B.J., Taub D.D., Trent J.M., Moon R.T., Bittner M., Weeraratna A.T. The Wnt5A/protein kinase C pathway mediates motility in melanoma cells via the inhibition of metastasis suppressors and initiation of an epithelial to mesenchymal transition. J Biol Chem 2007; 282(23): 17259–17271.
- Seifert J.R., Mlodzik M. Frizzled/PCP signalling: a conserved mechanism regulating cell polarity and directed motility. Nat Rev Genet 2007; 8(2): 126–138.
- Jones C., Chen P. Planar cell polarity signaling in vertebrates. Bioessays 2007; 29(2): 120–132.
- 19.Wallingford J.B., Habas R. The developmental biology of Dishevelled: an enigmatic protein governing cell fate and cell polarity. Development 2005; 132(20): 4421–4436.
- Evangelista M., Zigmond S., Boone C. Formins: signaling effectors for assembly and polarization of actin filaments. J Cell Sci 2003; 116(Pt 13): 2603–2611.
- Habas R., Kato Y., He X. Wnt/Frizzled activation of Rho regulates vertebrate gastrulation and requires a novel Formin homology protein Daam1. Cell 2001; 107(7): 843–854.
- Habas R., Dawid I.B., He X. Coactivation of Rac and Rho by Wnt/Frizzled signaling is required for vertebrate gastrulation. Genes Dev 2003; 17(2): 295–309.
- Li L., Yuan H., Xie W., Mao J., Caruso A.M., McMahon A., Sussman D.J., Wu D. Dishevelled proteins lead to two signaling pathways. Regulation of LEF-1 and c-Jun N-terminal kinase in mammalian cells. J Biol Chem 1999; 274(1): 129–134.
- Habas R., Dawid I.B. Dishevelled and Wnt signaling: is the nucleus the final frontier? J Biol 2005; 4(1): 2.
- Dorsky R.I., Moon R.T., Raible D.W. Control of neural crest cell fate by the Wnt signalling pathway. Nature 1998; 396(6709): 370–373.
- Wu J., Yang J., Klein P.S. Neural crest induction by the canonical Wnt pathway can be dissociated from anterior-posterior neural patterning in Xenopus. Dev Biol 2005; 279(1): 220–232.
- Vallin J., Thuret R., Giacomello E., Faraldo M.M., Thiery J.P., Broders F. Cloning and characterization of three Xenopus slug promoters reveal direct regulation by Lef/beta-catenin signaling. J Biol Chem 2001; 276(32): 30350–30358.
- LaBonne C., Bronner-Fraser M. Neural crest induction in Xenopus: evidence for a two-signal model. Development 1998; 125(13): 2403–2414.
- Sakai D., Tanaka Y., Endo Y., Osumi N., Okamoto H., Wakamatsu Y. Regulation of Slug transcription in embryonic ectoderm by beta-catenin-Lef/Tcf and BMP-Smad signaling. Dev Growth Differ 2005; 47(7): 471–482.
- De Calisto J., Araya C., Marchant L., Riaz C.F., Mayor R. Essential role of non-canonical Wnt signalling in neural crest migration. Development 2005; 132(11): 2587–2597.
- Ikeya M., Lee S.M., Johnson J.E., McMahon A.P., Takada S. Wnt signalling required for expansion of neural crest and CNS progenitors. Nature 1997; 389(6654): 966–970.
- Fathke C., Wilson L., Shah K., Kim B., Hocking A., Moon R., Isik F. Wnt signaling induces epithelial differentiation during cutaneous wound healing. BMC Cell Biol 2006; 7: 4.
- Larue L., Beermann F. Cutaneous melanoma in genetically modified animals. Pigment Cell Res 2007; 20(6): 485–497.
- Larue L., Luciani F., Kumasaka M., Champeval D., Demirkan N., Bonaventure J., Delmas V. Bypassing melanocyte senescence by beta-catenin: a novel way to promote melanoma. Pathol Biol (Paris) 2009; 57(7–8): 543–547.
- Dang C.V. c-Myc target genes involved in cell growth, apoptosis and metabolism. Mol Cell Biol 1999; 19(1): 1–11.
- Kuphal S., Lodermeyer S., Bataille F., Schuierer M., Hoang B.H., Bosserhoff A.K. Expression of Dickkopf genes is strongly reduced in malignant melanoma. Oncogene 2006; 25(36): 5027–5036.
- Yamaguchi Y., Morita A., Maeda A., Hearing V.J. Regulation of skin pigmentation and thickness by Dickkopf 1 (DKK1). J Investig Dermatol Symp Proc 2009; 14(1): 73–75.
- Mikheev A.M., Mikheeva S.A., Rostomily R., Zarbl H. Dickkopf-1 activates cell death in MDA-MB435 melanoma cells. Biochem Biophys Res Commun 2007; 352(3): 675–680.
- Haqq C., Nosrati M., Sudilovsky D., Crothers J., Khodabakhsh D., Pulliam B.L., Federman S., Miller J.R., Allen R.E., Singer M.I., Leong S.P., Ljung B.M., Sagebiel R.W., Kashani-Sabet M. The gene expression signatures of melanoma progression. Proc Natl Acad Sci USA 2005; 102(17): 6092–6097.
- Lin Y.C., You L., Xu Z., He B., Yang C.T., Chen J.K., Mikami I., Clement G., Shi Y., Kuchenbecker K., Okamoto J., Kashani-Sabet M., Jablons D.M. Wnt inhibitory factor-1 gene transfer inhibits melanoma cell growth. Hum Gene Ther 2007; 18(4): 379–386.
- Delmas V., Beermann F., Martinozzi S., Carreira S., Ackermann J., Kumasaka M., Denat L., Goodall J., Luciani F., Viros A., Demirkan N., Bastian B.C., Goding C.R., Larue L. Beta-catenin induces immortalization of melanocytes by suppressing p16INK4a expression and cooperates with N-Ras in melanoma development. Genes Dev 2007; 21(22): 2923–2935.
- Tsao H., Bevona C., Goggins W., Quinn T. The transformation rate of moles (melanocytic nevi) into cutaneous melanoma: a population-based estimate. Arch Dermatol 2003; 139(3): 282–288.
- Chien A.J., Moore E.C., Lonsdorf A.S., Kulikauskas R.M., Rothberg B.G., Berger A.J., Major M.B., Hwang S.T., Rimm D.L., Moon R.T. Activated Wnt/beta-catenin signaling in melanoma is associated with decreased proliferation in patient tumors and a murine melanoma model. Proc Natl Acad Sci USA 2009; 106(4): 1193–1198.
- Takahashi Y., Nishikawa M., Suehara T., Takiguchi N., Takakura Y. Gene silencing of beta-catenin in melanoma cells retards their growth but promotes the formation of pulmonary metastasis in mice. Int J Cancer 2008; 123(10): 2315–2320.
- Weeraratna A.T., Jiang Y., Hostetter G., Rosenblatt K., Duray P., Bittner M., Trent J.M. Wnt5a signaling directly affects cell motility and invasion of metastatic melanoma. Cancer Cell 2002; 1(3): 279–288.
- Bittner M., Meltzer P., Chen Y., Jiang Y., Seftor E., Hendrix M., Radmacher M., Simon R., Yakhini Z., Ben-Dor A., Sampas N., Dougherty E., Wang E., Marincola F., Gooden C., Lueders J., Glatfelter A., Pollock P., Carpten J., Gillanders E., Leja D., Dietrich K., Beaudry C., Berens M., Alberts D., Sondak V. Molecular classification of cutaneous malignant melanoma by gene expression profiling. Nature 2000; 406(6795): 536–540.
- O’Connell M.P., Fiori J.L., Xu M., Carter A.D., Frank B.P., Camilli T.C., French A.D., Dissanayake S.K., Indig F.E., Bernier M., Taub D.D., Hewitt S.M., Weeraratna A.T. The orphan tyrosine kinase receptor, ROR2, mediates Wnt5A signaling in metastatic melanoma. Oncogene 2010; 29(1): 34–44.










