Pathomorphological Study of Phototoxicity of Genetically-Encoded Photosensitizer KillerRed on Animal Tumors
The study of phototoxic effects of KillerRed in experimental tumors enables to understand the mechanisms of action of genetically-encoded photosensitizers and choose the directions for photodynamic therapy (PDT) development.
The aim of the investigation was to study pathomorphological characteristics of the protein KillerRed effect on animal tumors in laser exposure modes close to those used in photodynamic therapy with chemically synthesized photosensitizers.
Materials and Methods. The study was carried out on immunodeficient nude mice with subcutaneously implanted tumor HeLa (human cervical cancer). Tumor cells stably expressed genetically-encoded photosensitizer KillerRed in chromatin (KillerRed-Н2B). Control tumors were HeLa tumors without KillerRed. Tumor growth and fluorescence intensity change were observed in the process of radiation in vivo using fluorescence imaging on IVIS-Spectrum Sistem (Caliper Life Sciences, USA). Tumors were exposed to laser radiation at wavelength of 593 nm, with fluence of 180 J/cm2 three times every other day. 24 h after treatment the tumors were studied using confocal fluorescence microscopy and were analyzed histologically.
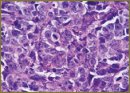
Results. Fluorescence imaging techniques in vivo and ex vivo after laser treatment of the tumors revealed the bleaching of KillerRed that was the criterion for selection of treatment regimen, and indicated photodynamic reaction. In acute post-irradiationperiod the tumors expressing KillerRed were found to have signs of tissue destruction. Along with moderate, reversible dystrophic changes such as enlargement and reduction in cell size and cytoplasm vacuolization, irreversible damage signs were revealed in most cells — damage of cell and nuclear membrane integrity.
The performed study showed for the first time the possibility in principle of tumor photodamage using phototoxic protein KillerRed in laser exposure in photodynamic therapy regimen.
- Kessel D. Photodynamic therapy of neoplastic disease. Vol. 2. CRC Press; 1990; 280 p.
- Hasan T., Ortel B., Moor A.C.E., Pogue B.W. Photodynamic therapy of cancer. In: Cancer medicine. Edited by Kufe D.W., et al. Hamilton (ON): BC Decker; 2003. p. 605–620.
- Gel’fond M.L. Fotodinamicheskaya terapiya v onkologii [Photodynamic therapy in oncology]. Prakticheskaya onkologiya — Practical Oncology 2007; 8(4): 204–210.
- Stepp H., Beck T., Pongratz T., et al. ALA and malignant glioma: fluorescence-guided resection and photodynamic treatment. J Environ Pathol Toxicol Oncol 2007; 26(2): 157–164.
- Cengel K.A., Glatstein E., Hahn S.M. Intraperitoneal photodynamic therapy. In: Peritoneal carcinomatosis. Ceelen W.P. (editor). Springer (US); 2007; p. 493–514.
- Berg K., Selbo P.K., Weyergang A., et al. Porphyrin-related photosensitizers for cancer imaging and therapeutic applications. J Microsc 2005; 218 (Pt 2): 133–147.
- Josefsen L.B., Boyle R.W. Photodynamic Therapy and the development of metal-based photosensitisers. Met Based Drugs 2008; 2008: 276109.
- Smirnova Z.S., Oborotova N.A., Makarova O.A., Orlova O.L., Polozkova A.P., Kubasova I.Yu., et al. Efficiency and pharmacokinetics of photosense: a new liposomal photosensitiser formulation based on aluminum sulfophthalocyanine. Pharm Chem J 2005; 39(7): 341–344.
- Gomer C.J., Ferrario A. Tissue distribution and photosensitizing properties of mono-L-aspartyl chlorin e6 in a mouse tumor model. Canc Res 1990; 50: 3985–3990.
- Castano A.P., Demidova T.N., Hamblin M.R. Mechanisms in photodynamic therapy. Part three. Photosensitizer pharmacokinetics, biodistribution, tumor localization and modes of tumor destruction. Photodiagnosis and Photodynamic Therapy 2005; 2(2): 91–106.
- Mroz P., Yaroslavsky A., Kharkwal G.B., and Hamblin M.R. Cell death pathways in photodynamic therapy of cancer. Cancers 2011; 3: 2516–2539.
- Nowis D., Makowski M., Stokіosa T., et al. Direct tumor damage mechanisms of photodynamic therapy. Acta Biochim Pol 2005; 52(2): 339–352.
- Oleinick N.L., Morris R.L., Nieminen A.L. Photodynamic therapy-induced apoptosis. In: Apoptosis, senescence and cancer. Gewirtz D.A., Holt S.E., Grant S. (editors). Totowa: Humana Press Inc.; 2007; 599 p.
- Krammer B. Vascular effects of photodynamic therapy. Anticancer Res 2001; 21(6B): 4271-4277.
- Bulina M.E., Chudakov D.M., Britanova O.V., Yanushevich Y.G., Staroverov D.B., Chepurnykh T.V., Merzlyak E.M., Shkrob M.A., Lukyanov S., Lukyanov K.A. A genetically encoded photosensitizer. Nat Biotechnol 2006; 24(1): 95–99.
- Pletnev S., Gurskaya N.G., Pletneva N.V., Lukyanov K.A., Chudakov D.M., Martynov V.I., Popov V.O., Kovalchuk M.V., Wlodawer A., Dauter Z., Pletnev V. Structural basis for phototoxicity of the genetically encoded photosensitizer KillerRed. J Biol Chem 2009; 284(46): 32028–32039.
- Vegh R.B., Solntsev K.M., Kuimova M.K., Cho S., Liang Y., Loo B.L., Tolbert L.M., Bommarius A.S. Reactive oxygen species in photochemistry of the red fluorescent protein ‘‘Killer Red”. Chem Comm 2011; 47(17): 4887–4889.
- Bulina M.E., Lukyanov K.A., Britanova O.V., Onichtchouk D., Lukyanov S., Chudakov D.M. Chromophore-assisted light inactivation (CALI) using the phototoxic fluorescent protein KillerRed. Nat Protoc 2006; 1(2): 947–953.
- Serebrovskaya E.O., Gorodnicheva T.V., Ermakova G.V., Solovieva E.A., Sharonov G.V., Zagaynova E.V., Chudakov D.M., Lukyanov S., Zaraisky A.G., Lukyanov K.A. Light-induced blockage of cell division with a chromatin-targeted phototoxic fluorescent protein. Biochem J 2011; 435(1):65–71.
- Bonnett R., Martnez G. Photobleaching of sensitisers used in photodynamic therapy. Tetrahedron 2001; 57(47): 9513–9547.
- Shirmanova M.V., Serebrovskaya E.O., Lukyanov K.A., Snopova L.B., Sirotkina M.A., Prodanetz N.N., Bugrova M.L., Minakova E.A., Turchin I.V., Kamensky V.A., Lukyanov S.A., Zagaynova E.V. Phototoxic effects of fluorescent protein KillerRed on tumor cells in mice. J Biophotonics 2012 Jun 13. doi: 10.1002/jbio.201200056.
- Cabrera G., Porvasnik S.L., DiCorleto P.E., et al. Intra-arterial adenoviral mediated tumor transfection in a novel model of cancer gene therapy. Mol Canc 2006; 5: 32.
- Kamimura K., Suda T., Zhang G., Liu D. Advances in gene delivery systems. Pharmaceut Med 2011; 25(5): 293–306.
- Berg K., Selbo P.K., Prasmickaite L., et al. Photochemical Internalization: a new tool for gene and oligonucleotide delivery. Top Curr Chem 2010; 296: 251–281.










