In vivo and in vitro Development and Study of Osteoplastic Material Based on Hydroxyapatite, Poly-3-Hydroxybutyrate and Sodium Alginate Composition
The aim of the investigation was to develop a new synthetic material based on poly-3-hydroxybutyrate, sodium alginate and hydroxyapatite in the form of a paste and study in vitro and in vivo its efficiency when repairing bone defects.
Materials and Methods. For a paste we used poly-3-hydroxybutyrate (PHB) developed microbiologically, with molecular weight of 52 kDa, hydroxyapatite (HAP) and sodium alginate (Sigma-Aldrich, Germany).
Results. A comparative study of implantation results of HAP–PHB and xenogeneic osseous non-demineralized collagen with a defect covered by PHB membrane showed that by day 180 critical bone defect healed completely in a group with HAP–PHB implantation. Despite the fact that cortical plate formed in a group of patients with implantation of xenogeneic osseous non-demineralized collagen, chronic productive inflammation results in osseous tissue rarefaction and fibrosis formation in interjoist that can have a negative effect on mechanical properties of the bone.
Conclusion. The obtained biomaterial based on composite microparticles from PHB and HAP in alginate gel can be used as a filling agent to correct bone tissue defects, since in its structure there are combined solid support elements and a substance able to maintain optimal microenvironment for cell culture.
Despite advanced technologies in bone bioengineering, development and introduction of novel osteoplastic materials into clinical practice still remains a topical issue. Most osteoplastic materials contain xenogeneic and/or allogeneic components with osteoconductive or in some cases osteoinductive properties. And despite high-scale purification the materials manufactured from animal tissues can cause immune response of a recipient and transfer some infections.
Synthetic materials are various composites, ceramics cements based on synthetic calcium hydroxyapatite, calcium phosphate and their combinations [1]. These materials also demonstrate osteoconductive properties. However, there are suppositions about osteoinductive properties of calcium hydroxyapatite nanoparticles [2, 3].
The form of material was found to be also of importance for clinical application. Powdered osteoplastic materials are uneasy for a filling of jaw bone damages with irregular shapes (cystic cavities, alveolar sockets, maxillary sinus in maxillary sinus lift surgery), need long time procedures and reveal difficulties to meet the requirement for homogeneity in bone cavity filling. Paste- or gel-like osteoplastic materials are free from the above mentioned drawbacks.
Engineering of such biomaterials has to consider both compositions and their components as it is important to obtain optimal biomaterial properties. Current polymer bioengineering proposes novel biodegradable and biocompatible polymers such as poly-3-hydroxybutirate (PHB) and alginate as main components for design of bone reconstructive systems. PHB is biodegradable polyester obtained by biotechnology [4–7]. It demonstrates such valuable for clinical applications properties as biocompatibility [6–8] and biodegradability within living tissues without toxic product formation [9–11]. In this regard, PHB is used for manufacturing a variety of medical devices for in hernioplasty, stomatology, cardiac surgery, orthopedy and some other clinical areas providing its application in maxillofacial surgery for bone regeneration [12, 13]. Alginate-based hydrogels are also frequently used in tissue engineering. Alginates are polysaccharides derived from sea algae and belong to the family of hyaluronic acid and mannonic acids. Sequence of components in alginates is different, but they can form insoluble gel in presence of bivalent cations. Reversible gel formation occurs in alginates due to ion interactions. Alginate gels also demonstrate a high biocompatibility [14]. However, the mentioned biomaterials when used separately sometimes do not meet the requirements for advanced bone regeneration systems. Therefore, the development of novel synthetic materials with optimized composition and formulation for replacement and restoration of bone damages is to be continued.
The aim of the investigation was the engineering of a novel slurred synthetic material based on poly-3-oxybutirate, alginate and hydroxyapatite with following in vitro and in vivo study of product efficacy in restoration of bone damage.
The study tasks were the following: 1) to develop technology for production of a paste-like synthetic bone replacing material containing composite microparticles with hydroxyapatite and poly-3-oxybutirate (HAP–PHB) within alginate gel; 2) to study biocompatibility of developed product on cell cultures; 3) to assess the product effectiveness in regeneration of bone damages on animals in experiments.
Materials and Methods. 52 kDa poly-3-oxybutirate obtained microbiologically, hydroxyapatite and sodium alginate (Sigma-Aldrich, Germany) were used for making a paste-like product.
Biosynthesis and PHB-separation. Azotobacter chroococcum 7Б strain-producers with PHB-overproduction (up to 80% of PHB in cell dry mass) were used for polymer synthesis. PHB-overproduction was achieved in Azotobacter cultures by their cultivation at 30°C on Berk`s media in presence of carbon source in excess concentration (g/L): МgSO4·7H2O — 0.4; FeSO4·7H2O — 0.01; Na2MoO4·2H2O — 0.006; sodium citrate — 0.5; CaCl2 — 0.1; K2HPO4·3H2O — 1.05; KH2PO4 — 0.2; saccharose — 40. Sodium acetate (12 g/L) was added to cultural media to reduce molecular mass [4].
Isolation of the polymer from biomass and product purification included the following steps: 1) isolation of PHB from bacterial mass by chloroform extraction by shaking at 37°С for 12 h; 2) filtration of PHB-solution for disposal of cell remnants; 3) isolation of PHB from chloroform solution by its precipitation with isopropyl alcohol; 4) multiply dissolving in chloroform with following precipitation by isopropanol; 5) drying at 60°С.
Preparation of microparticles. Microparticles with encapsulated hydroxyapatite HAB–PHB were generated using technology of two-stage emulsification W/V/W: hydroxyapatite emulsion in 3% polymer solution (1:5 w/w) was added gradually to the 100 ml of 1.5% polyvinyl alcohol water solution, mixing by overhead stirrer (R2R 2021, Heidolph, Germany) at 600–800 rpm. After full evaporation of organic solvent, microparticles were isolated by centrifugation (10 min at 4400 rpm) and triple washed with distillated water until full disposal of the emulsifier.
Paste preparation. The necessary formulation of the product was made by intensive emulsification of microparticles in 1% sodium alginate solution in the ratio 60:40 correspondingly. Final emulsion should be hermetically packed to avoid drying and therefore change of biopolymer concentration as well as mechanical characteristics of the product.
Study of the paste biocompatibility on cell culture in vitro. In vitro assessment of paste biocompatibility was carried out by adding of calcium ions (CaCl2) to alginate sol for enhancement its stability. Alginate gel without polymeric microparticles was used as control. The study was carried out on cultures of green monkey’s fibroblasts COS-1 (Biolot, Russia). The cells were cultivated in DMEM (Dubecco’s Modified Eagle Medium; Invitrogen, USA) glucose-enriched (4.5 g/L) including 10% fetal calf serum, penicillin (100 U/ml) and streptomycin (100 µg/ml). The cells were incubated at 37°C in atmosphere containing 5% CO2, cultural media being renewed daily. Six 5×5 mm samples of the study product and controls were placed in a 96-well microtiter plate, each sample being added by cell suspension (4000 cells per sample). The plates were incubated 2, 5, 7, 9 and 12 days. Fibroblasts were isolated from samples by washing with phosphate buffered saline (Serva, Germany) containing 0.05% trypsin + 0.02% EDTA and then counted using Goryaeb chamber. Biocompatibility of polymer systems was assessed in a standard cell viability assay XTT Cell Proliferation Kit (Biological Industries, Israel). The number of viable cells was evaluated by a standard curve for ХТТ [8].
Microscopy. Primary examination of the microparticle properties was carried out by light microscopy (microscope Biomed-1 v.2, Biomed, Russia supplied with digital eyepiece MYscope 300M Webbers, Taiwan). Microphotographs were taken by scanning electron microscopy in both electron and ion beam radiation using FEI-SMA-QUANTA 200 and SMA QUANTA FEG (LTD “Systems for microscopy and analysis”, Russia).
Assessment of the product’s efficacy in regeneration of bone damages on animals in experiment. The work was performed in accordance with ethical principles established by European Convention for the protection of vertebrata used for experimental and other scientific purposes (the Convention was passed in Strasburg, March, 18, 1986, adopted in Strasburg, June, 15, 2006) and approved by Ethics Committee of Nizhny Novgorod State Medical Academy.
The study included two stages. The first stage was performed using 10 mature Wistar rats. The animals were kept in vivarium of the NNSMA Central Research Laboratory at 22°С and 15 hours of daylight. The rats were anesthetized intravenously with Zoletil 5 mg/kg; then skin incision, skeletonization of femoral bone were performed. A cylindrical cavity (d=2 mm) was formed in bone and filled with study product, the wound was stitched up by layers. Rats were sacrificed 2 weeks after the operation, their bone fragments of study interest were isolated, fixed in 10% formaldehyde solution and submitted to histological investigation.
The second stage was performed using 6 mature minipigs. These animals were kept in vivarium of Scientific Centre of Biomedical Technologies, Russian Federal Medical and Biological Agency at 22°С and 15 hours of daylight. Minipig mandibles on the right and left side were skeletonized under local anesthesia and IV Zoletil 5 mg/kg. 4 cylindrical cavities (10×10×5 mm) were formed in each mandible. Group I (experimental) animals (n=3) were treated as follows: bone defect was filled by the material under study and covered by PHB-membrane. Surgical wounds were stitched. Group II (control) animals (n=3) were treated as follows: crumb of xenogeneic bone non demineralized collagen was administered in each bone defect and covered by PHB-membrane. Surgical wounds were stitched up by layers. Minipigs were sacrificed 1, 3 and 6 months after the operation, their bone fragments of study interest (4 samples from each animal) were isolated, fixed in 10% formaldehyde solution and submitted to histological investigation.
Results and Discussion
Product characteristics. A developed technique enables to vary the size of microparticles up to 300–400 µm. An average size of microparticles used in the study was 52±11 µm. This size was chosen as optimal due to its adequacy with cells, which can attach and grow on microparticles using them as support substrate.
Scanning electron microscopy revealed microparticles to have high-porous surface containing twisted polymer bands (Fig. 1). Previously we showed that a rough surface promoted attachment and growth of cells on the microparticles [8].
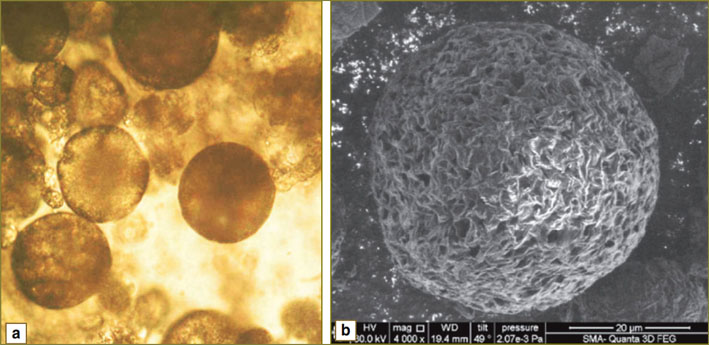 Fig. 1. Microphoos of composite PHB and HAP microparticles taken using light microscopy (a) and electron microscopy (b); (×4000 magnification) Fig. 1. Microphoos of composite PHB and HAP microparticles taken using light microscopy (a) and electron microscopy (b); (×4000 magnification)
|
Biopolymer product under study is a paste containing PHB and HAP microparticles in alginate sol (Fig. 2). The paste is ideally suited for filling bone damages due to its structure.
 Fig. 2. The biopolymer paste appearance Fig. 2. The biopolymer paste appearance
|
The photo taken by scanning electron microscopy (Fig. 3) demonstrates a cluster of bonded microparticles submerged into alginate gel. Hydroxyapatite crystals on the surface of microparticles are clearly seen. It is very important property of the product, because such structure should induce the generation of bone tissue due to osteoinduction of hydroxyapatite located on microparticles surface and released in biodegradation.
 Fig. 3. Biopolymer paste containing HAP–PHB microparticles in alginate gel. Scanning electron microscopy (×2000 magnification) Fig. 3. Biopolymer paste containing HAP–PHB microparticles in alginate gel. Scanning electron microscopy (×2000 magnification)
|
Biocompatibility. Sodium alginate has been reported [e.g., 14] to be a good substrate for cell growth. We found the number of viable cells in alginate gel to remain unchanged (Fig. 4). This finding characterizes alginate gel as a good environment for maintaining cell viability, but insufficient — for cell growth and proliferation. Whencultivating cells using the designed product we noticed that in course of time the number of living cells starts increasing. The finding demonstrates the improvement of cultural environment for cell growth. The main difference of designed paste from genuine alginate gel is the presence of solid microparticles, 52±11 µm in size, i.e. comparable with cell size (25–35 µm). So, we may conclude that these particles exhibit good adherence and cells use them assubstrate for bonding and further active growth. Therefore, structure of the designed biopolymer product includes elements of solid substrate and the substance able to maintain optimal microenvironment for cell culture.
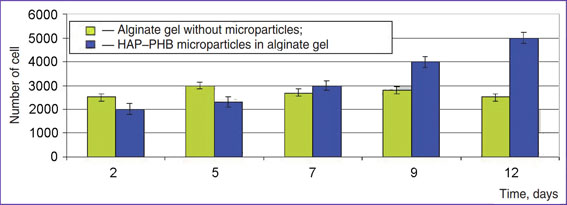 Fig. 4. Growth of cell culture COS-1 on the polymer product Fig. 4. Growth of cell culture COS-1 on the polymer product
|
In vivo study results. Study results related to the regeneration of bone damages in rats showed implantation of a designed product containing HAP–PHB to improve significantly the conditions for bone tissue regenerationin experimental models of rat femoral bone damage; the material has osteoinductive activity that is indicated by expansion of newly formed bone tissue along the product surface. The material does not cause inflammation and is reabsorbed by giant cells of foreign bodies, as well as does not change the direction of regeneration front that is indicated by the restoration of anatomical structure of the damaged femoral cortex (Fig. 5, 6).
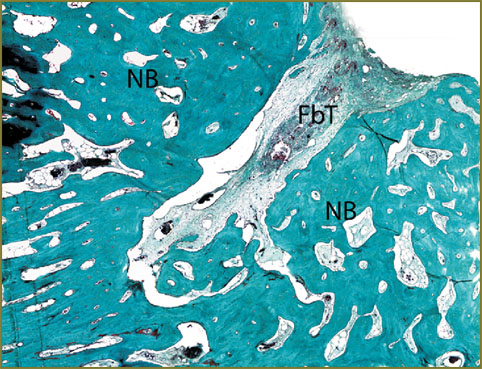
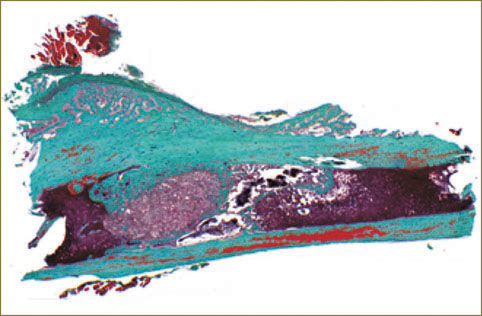 Fig. 5. Histotopogram. Bone regeneration 30 days after implantation of HAP–PHB material into a rat femoral bone defect. The material looks like a conglomerate surrounded by bone tissue in intramedullary canal
Fig. 5. Histotopogram. Bone regeneration 30 days after implantation of HAP–PHB material into a rat femoral bone defect. The material looks like a conglomerate surrounded by bone tissue in intramedullary canal
Fig. 6. Generation of chondroid and its maturation. Masson–Goldner staining, ×200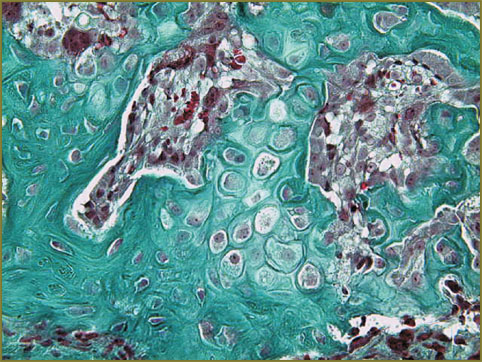
|
The analysis of bone tissue samples on day 30, 90 and 180 after simulation of minipig’s mandibular wound, 1 cm in diameter, and filling bone defects by HAP–PHB material, with covered with PHB-membrane showed bone tissue regeneration to start in maternal bone and expand from peripheral areas to the center of reclaimed product. In the connective tissue in central area of regeneration there were the remnants of the osteoplastic material with poorly differentiated connective tissue around them. On day 180 cortical plate still contained a rounded funnel-shaped defect filled with connective tissue. A lot of active osteoblasts were seen on the surface of bone trabeculae. At the boundary of fibrous and bone tissue there were foci of reticulofibrous bone tissue (Fig. 7).
Histological study of bone tissue samples 180 days after implantation of xenogeneic bone non-demineralized collagen in bone defects covered by PHB-membrane revealed that despite normal regeneration of cortical plate, in underlying areas there were foci of fibrous connective tissue with osteoporosis marked by fibrosis of intertrabecular space invading in all directions along the marrowy canal.
The study of histological preparations also showed the use of this material to result in chronic granulomatous inflammation for at least 90 days after implantation and, with the development of intertrabecular fibrosis by day 180 (Fig. 8).
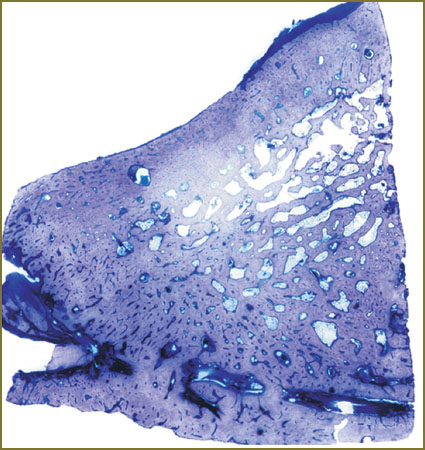 Fig. 8. Termination of osteogenesis 90 days after implantation of HAP–PHB osteoplastic material and covering by PHB-membrane. Alcian blue staining; ×32 Fig. 8. Termination of osteogenesis 90 days after implantation of HAP–PHB osteoplastic material and covering by PHB-membrane. Alcian blue staining; ×32
|
Thus, a comparative analysis of the results ofimplantation of HAP–PHB biopolymer product and xenogeneic bone non-demineralized collagen covered by PHB-membrane showed a critical bone defect to be completely regenerated by day 180 after HAP–PHB implantation. Despite the fact that cortical plate formed in a group of patients with implantation of xenogeneic bone non-demineralized collagen, chronic productive inflammation results in bone tissue rarefaction and fibrosis formation in intertrabecular space that can have a negative effect on mechanical properties of the bone.
Conclusion. Osteoplastic biopolymer designed on the basis of composite microparticles from hydroxyapatite and poly-3-oxybutirate in alginate gel can be used as a filling material to correct bone tissue defects, since its structure combines the elements of a solid substrate and a substance capable to provide optimal microenvironment for cell culture. This material demonstrates osteoinductive properties and is completely replaced by bone tissue in the process of biological resorption.
Study Funding. This work was funded by the authors.
Conflict of Interests. The authors have no conflict of interests to disclose.
References
- Ivanov S.Y., Mukhametshin R.F., Muraev A.A., Bonartsev A.P., Rabova V.M. Sinteticheskie materialy, ispol'zuemye v stomatologii dlya zameshcheniya defektov kostnoy tkani [Synthetic materials used in dentistry to fill bone defects]. Sovremennye problemy nauki i obrazovaniya — Modern Scientific and Educational Problems 2013; 1. URL:www.science-education.ru/107-8345.
- Li B., Liao X., Zheng L., Zhu X. Wang Z., Fan H., Zhang X. Effect of nanostructure on osteoinduction of porous biphasic calcium phosphate ceramics. Acta Biomater 2012 Oct; 8(10): 3794–3804, http://dx.doi.org/10.1016/j.actbio.2012.06.021.
- Götz W., Lenz S., Reichert C., Henkel K.O., Bienengröber V., Pernicka L., Gundlach K.K., Gredes T., Gerber T., Gedrange T., Heinemann F. A preliminary study in osteoinduction by a nano-crystalline hydroxyapatite in the mini pig. Folia Histochem Cytobiol 2010 Dec; 48(4): 589–596, http://dx.doi.org/10.2478/v10042-010-0096-x.
- Myshkina V.L., Nikolaeva D.A., Makhina T.K., Bonartsev A.P., Bonartseva G.A. Vliyanie usloviy kul’tivirovaniya na molekulyarnuyu massu poli-3-gidroksibutirata, sinteziruemogo Azotobacter chroococcum 7 B [The effect of culture conditions on molecular weight of poly-3-hydroxybutyrate synthesized by Azotobacter chroococcum 7 B]. Prikladnaya biokhimiya i mikrobiologiya — Applied Biochemistry and Microbiology 2008; 44(5): 533–538.
- Myshkina V.L., Ivanov E.A., Nikolaeva D.A., Makhina T.K., Bonartsev A.P., Filatova E.V., Ruzhitskiy A.O., Bonartseva G.A. Biosintez sopolimera poli-3-gidroksibutirata-3-gidroksivalerata shtammom Azotobacter chroococcum 7 B [Biosynthesis of poly-3-hydroxybutyrate-3-hydrozyvalerate copolymer by Azotobacter chroococcum 7B strain]. Prikladnaya biokhimiya i mikrobiologiya — Applied Biochemistry and Microbiology 2010; 46(3): 1–8.
- Bonartsev A.P., Yakovlev S.G., Zharkova I.I., Boskhomdzhiev A.P., Bagrov D.V., Myshkina V.L., Makhina T.K., Kharitonova E.P., Samsonova O.V., Feofanov A.V., Voinova V.V., Zernov A.L., Efremov Yu.M., Bonartseva G.A., Shaitan K.V., Kirpichnikov M.P. Cell attachment on poly(3-hydroxybutyrate)-poly(ethylene glycol) copolymer produced by Azotobacter chroococcum 7B. BMC Biochemistry 2013: 14: 12.
- Bonartsev A., Yakovlev S., Boskhomdzhiev A., Zharkova I., Bagrov D., Myshkina V., Mahina T., Charitonova E., Samsonova O., Zernov A., Zhuikov V., Efremov Yu., Voinova V., Bonartseva G., Shaitan K. The terpolymer produced by Azotobacter chroococcum 7B: effect of surface properties on cell attachment. PLoS ONE 2013; 8(2): e57200, http://dx.doi.org/10.1371/journal.pone.0057200.
- Zharkova I.I., Bonartsev A.P., Boskhomdzhiev A.P., Efremov Yu.M., Bagrov D.V., Makhina T.K., Myshkina V.L., Ivanov E.A., Voinova V.V., Yakovlev S.G., Zernov A.L., Filatova E.V., Andreeva N.V., Bonartseva G.A., Shaytan K.V. Vliyanie modifikatsii poli-3-oksibutirata polietilenglikolem na zhiznesposobnost’ kletok, kul’tiviruemykh na polimernykh plenkakh [The effect of polyethylenglycol modification poly-3-hydroxybutyrate on viability of cells cultured on polymer films]. Biomeditsinskaya khimiya — Biomedical Chemistry 2012; 58(5): 579–591.
- Boskhomdzhiev A.P., Bonartsev A.P., Makhina T.K., Myshkina V.L., Ivanov E.A., Bagrov D.V., Filatova E.V., Iordanskiy A.L., Bonartseva G.A. Sravnitel’noe izuchenie kinetiki biodegradatsii biopolimernykh sistem na osnove poli-3-oksibutirata [Comparative study of biodegradation kinetics of biopolymer systems based on poly-3-hydroxybutyrate]. Biomeditsinskaya khimiya — Biomedical Chemistry 2009; 55(6): 625–635.
- Boskhomdzhiev A.P., Bonartsev A.P., Ivanov E.A., Makhina T.K., Myshkina V.L., Bagrov D.V., Filatova E.V., Bonartseva G.A., Iordanskiy A.L. Gidroliticheskaya destruktsiya biopolimernykh sistem na osnove poli-3-oksibutirata. Kineticheskiy i strukturnyy aspekty [Hydrolytic destruction of biopolymer systems based on poly-3-hydroxybutyrate. Kinetic and structural aspects]. Plasticheskie massy — Plastic Masses 2009; 8: 13–18.
- Bonartsev A.P., Boskhomodgiev A.P., Iordanskii A.L., Bonartseva G.A., Rebrov A.V., Makhina T.K., Myshkina V.L., Yakovlev S.A., Filatova E.A., Ivanov E.A., Bagrov D.V., Zaikov G.E. Hydrolytic degradation of poly(3-hydroxybutyrate), polylactide and their derivatives: kinetics, crystallinity, and surface morphology. Molecular Crystals and Liquid Crystals 2012; 556(1): 288–300.
- Bonartsev A.P., Yakovlev S.G., Filatova E.V., Soboleva G.M., Makhina T.K., Bonartseva G.A., Shaytan K.V., Popov V.O., Kirpichnikov M.P. Prolongirovannoe vysvobozhdenie protivoopukholevogo lekarstvennogo veshchestva, paklitaksela, iz mikrosfer na osnove poli-3-oksibutirata [Prolonged release of antitumor agent, paclitaxel, from microspheres based on poly-3-hydroxybutyrate]. Biomeditsinskaya khimiya — Biomedical Chemistry 2011; 57(2): 232–240.
- Artsis M.I., Bonartsev A.P., Iordanskii A.L., Bonartseva G.A., Zaikov G.E. Biodegradation and medical application of microbial poly(3-hydroxybutyrate). Molecular Crystals and Liquid Crystals 2012; 555(1): 232–262.
- Biosovmestimye materialy [Biocompatible materials]. Pod. red. Sevast’yanova V.I., Kirpichnikova M.N. [Sevast’yanov V.I., Kirpichnikov M.N. (editors)]. Moscow; 2011.