Assessment of Cytotoxic Effect Mechanisms of Gas-Discharge Plasma Radiation
The aim of the investigation was to assess the mechanisms of cytotoxic effect of gas-discharge plasma radiation on lymphosarcoma and breast cancer cells.
Materials and Methods. The experiment was carried out on the strains of rat lymphosarcoma (LSR) and breast cancer (RMK1) cells. 4 ml of cell suspension at (4–6)·106/ml concentration was exposed to gas-discharge plasma radiation in various time modes. Plasma radiation was generated by impulse device with the following set characteristics: burst time — 100 µs, voltage — 11 kV, energy per pulse — 5.9·10-2 J, pulse frequency — 10 Hz. Cytotoxic effect of gas-discharge radiation was assessed using fluorescent dye Hoechst (Sigma ALDRICH, USA), Propidium iodide (Sigma ALDRICH, USA) and МТТ-test. Structural changes in cells were studied by electron microscopy. Cytoplasmic membrane condition was assessed by microviscosity change using a hydrophobic fluorescent probe pyrene (Sigma ALDRICH, USA). The level of oxidative processes was determined by fluorescence of bityrosine, tryptophan, glycated proteins and lipid peroxidation processes. The state of coenzymes was estimated by NAD(P)+/NAD(P)Н+Н+ and FAD+/FADН2. DNA cell damage degree was assessed by DNA-comet assay.
Results. 600-second radiation exposed to LSR and RMK1 cells was found to be a half-lethal dose. Such radiation causes significant changes in the structure of cytoplasmic and nuclear membranes, intracellular content, reduces microviscosity indices both in a lipid bilayer and in protein-lipid interaction area of LSR and RMK1 cells. Protein molecules of these cells undergo marked oxidative modification exposed to gas-discharge plasma radiation. No accumulation of lipid peroxidation products was recorded. The content of reduced NAD(P)Н+Н+ and oxidized FAD+ increases in the cells under study under plasma radiation. The number of cells with significantly damaged DNA increases up to 81% by 600th second of exposure. All changes were in direct relationship to time exposure duration.
Plasma technologies in biology and medicine is a dynamically developing direction, biomedical effects of plasma is being actively studied by researchers all over the world. Development of plasma devices generating pharmacological doses of reactive species, and standartization of plasma technologies can solve many problems and open up new prospects both in physiological, and biochemical researches, as well as in the development of new treatment modalities.
By now, there have been published a great deal of studies indicating biological effects of plasma, such as bactericidal, sporicidal, cytotoxic effects, and regeneration [1–3]. Due to the discovery of pro-apoptotic activity of plasma, researchers are actively studying the possibility to apply plasma technologies for antitumor therapy [4–6]. However, much less is known about the mechanisms of plasma cytotoxic effect, and the data obtained in numerous research laboratories turn out to be rather contradictory in relation to the contribution of various factors of plasma (ions, radicals, electromagnetic fields, plasma radiation) to bactericidal and cytotoxic effects that explains the urgent character of the studies in this field.
Radiation is the main operative factor of spark plasma. By now there have already been detected some reactive species of plasma radiation formed in gas phase and liquid, and found bactericidal, sporicidal and cytotoxic effects of plasma radiation; structural and functional changes of prokaryotic cells are being under study [7–9]. However, no complex study of structural and functional condition of eukaryotic cells after spark plasma exposure has been carried out so far.
The aim of the investigation was to assess the mechanisms of cytotoxic effect of gas-discharge plasma radiation on lymphosarcoma and breast cancer cells.
Materials and Methods. The experiment was carried out on the strains of rat lymphosarcoma (LSR) and breast cancer (RMK1) cells. LSR strain was obtained from an outbread rat that had been given 3.3-dichlorbenzidine, the tumor consisting of lymphoid cells of different size. RMK1 strain was received from breast ducts of outbread female rats; carcinoma occurring spontaneously and being presented by alveolar structures and atypical epithelial cells. Strains were obtained in N.N. Blokhin Russian Cancer Research Center (Moscow).
Spark plasma radiation was generated by impulse device Pilimin with the following characteristics: burst time — 100 µs, voltage — 11 kV, energy per pulse — 5.9·10−2J, pulse frequency — 10 Hz. The device was developed by I.M. Piskarev, a leading research worker of Skobeltsyn Institute of Nuclear Physics of Lomonosov Moscow State University in 2011.
For analysis the cells were resuspended in Hanks’ solution (Biolot, Russia) up to the concentration of (4–6)·106/ml. 4 ml of cell suspension was exposed to low-temperature gas-discharge plasma radiation in various time modes from 30 to 1800 s.
Cytotoxic effect of gas-discharge plasma radiation was assessed using fluorescent dye Hoechst (Sigma ALDRICH, USA), Propidium iodide (Sigma ALDRICH, USA) and МТТ-test [10, 11]. The measurements were made using fluorescent microscope Leica DMIL HC (Leica Microsystems, Germany) and spectrofluorometer Fluorat-02 Panorama (Lumex, Russia).
Structural changes in cells were studied by electron microscopy [12]. Electronic photos were taken using electron-transmission microscope Morgagni 268D (FEI, USA). Video camera Mega View (Arecont Vision, USA) translated an image on a computer screen. The images were processed using AnalySIS program.
Cytoplasmic membrane condition was assessed by microviscosity changes using a hydrophobic fluorescent probe pyrene (Sigma ALDRICH, USA) [13]. The measurements were made using spectrofluorometer Fluorat-02 Panorama (Lumex, Russia).
Folch method was used to extract lipids from the material under study. Total lipids were determined using TOTAL LIPIDS BIO-LACHEMA-TEST (PLIVA-Lachema Diagnostica, Czech Republic). Lipid peroxidation intensity was estimated by relative concentrations of low-molecular unoxidized products, diene conjugates, molecules with cis- and trans-conjugated olefinic bonds, triene conjugates, malondialdehyde, Schiff’s bases [14–16]. The measurements were made by spectrofluorometer Fluorat-02 Panorama (Lumex, Russia).
Total protein concentration was determined by biuret method using TOTAL PROTEIN “FL-E” (VITAL DIAGNOSTICS, Russia). Protein oxidative modification was assessed by bityrosine fluorescence, non-enzymatic protein glycosylation products and tryptophan residues [17–19]. The measurements were made using spectrofluorometer Fluorat-02 Panorama (Lumex, Russia).
The condition of coenzymes was estimated by NAD(P)Н+Н+ and FAD+ fluorescence [20, 21]. The measurements were made using spectrofluorometer Fluorat-02 Panorama (Lumex, Russia).
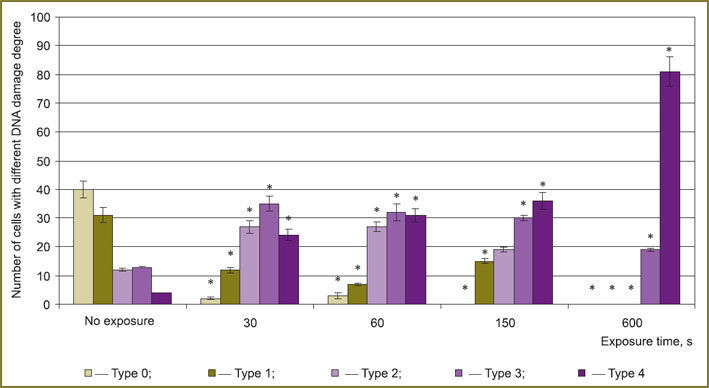
DNA cell damage degree was assessed by DNA-comet assay. To determine a proportion of cells with different DNA damage degree we assessed their distribution in groups depending on “DNA percentage in a comet tail” value: from 0 to 5%; from 5.1 to 10%; from 10.1 to 15%; from 15.1 to 20%; over 20% [22]. Gel-slides were analyzed on fluorescent microscope Leica DMIL HC (Leica Microsystems, Germany).
The findings of the experiments were processed using software package Excel и Statistica v.6.0.
Results and Discussion. 600-second radiation exposed to LSR and RMK1 cells was found to be a half-lethal dose (Fig. 1).
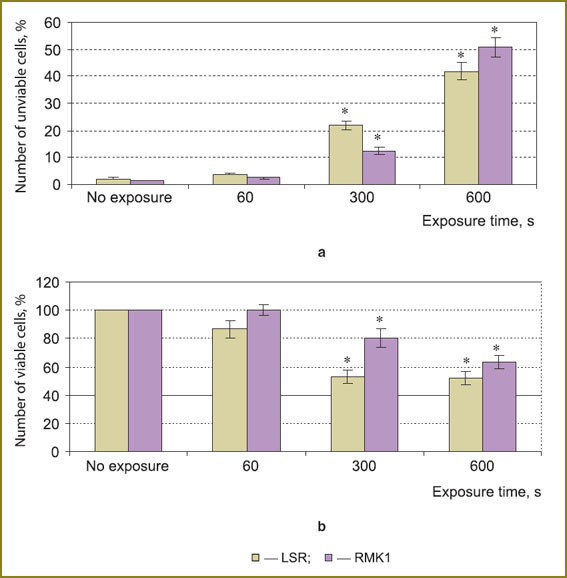 Fig. 1. The number of unviable (а) and viable (b) LSR and RMK1 cells after plasma radiation exposure; * — statistically significant in relation to an untreated series, p<0.05 Fig. 1. The number of unviable (а) and viable (b) LSR and RMK1 cells after plasma radiation exposure; * — statistically significant in relation to an untreated series, p<0.05
|
Then we studied structural changes in cells under gas-discharge plasma radiation. LSR cells of control series (untreated) were found to be rounded with intact cytoplasmic and nuclear membrane. Cellular content after being exposed to plasma radiation was seen to have rarefaction. With exposure time increase, the integrity of cytoplasmic and nuclear membrane damaged, and pores formed (Fig. 2).
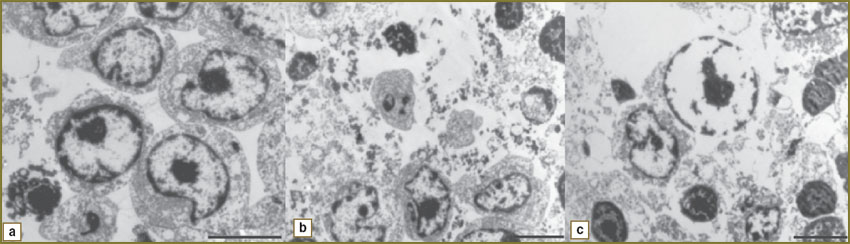 |
Fig. 2. Eletronic photographs of LSR cells after discharge plasma radiation exposure: а — control; b — 15-minute exposure; c — 30-minute exposure (length size — 5 µm) |
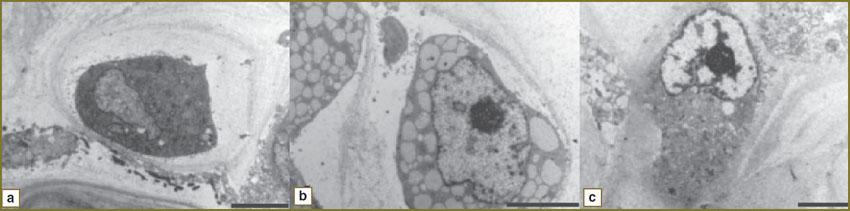
Most control RMK1 cells were ovoid, with a large nucleus, nuclear chromatin having fine grained structure. Nucleoli were large. 15-minute plasma radiation exposure caused intracellular content vacuolization and nuclear membrane deformity. 30-minute exposure resulted in nuclear membrane ruptures (Fig. 3).
|
Fig. 3. Electronic photographs of RMK1 cells after discharge plasma radiation exposure: а — control; b — 15-minute exposure; c — 30-minute exposure (length size — 5 µm) |
Thus, plasma radiation causes certain abnormalities in the structure of cytoplasmic and nuclear membranes and intracellular content of LSR and RMK1 cells, the significance of these abnormalities being in direct relationship to exposure time duration.
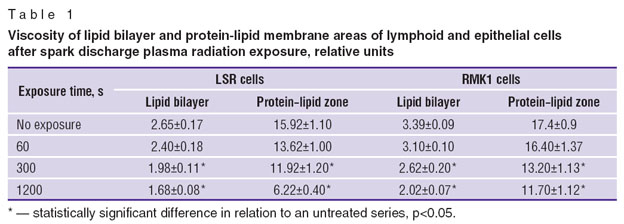
The study of the cell cytoplasmic membrane condition showed microviscosity parameters to decrease with exposure duration increase: both in lipid bilayer — by 1.6–1.7 times, and in the area of protein–lipid interaction — by 1.5–2.6 times (Table 1). Microviscosity change is known to produce a significant effect on membrane-bound processes, such as permeability for ions and molecules participating in cell metabolism, and on the action of membrane enzymes and receptor systems [23] that is likely to have an impact on cell viability as well.
|
Table 1. Viscosity of lipid bilayer and protein-lipid membrane areas of lymphoid and epithelial cells after spark discharge plasma radiation exposure, relative units |
Membrane microviscosity is a total index, which depends both on protein component state, and the state of lipid molecules [23]. In this regard, next we studied oxidation processes of proteins and lipids.
The study of lipid peroxidation in LSR and RMK1 cells under plasma radiation revealed no changes in relation to concentrations of primary, secondary and end products. These findings are consistent with data we obtained in 2012 and 2013 [9, 24]. The researches showed that relative concentrations of diene and triene conjugates did not increase, and the intensity of lipid peroxidation processes of bacterial cells decreased after liposome suspension of common lipids, cholesterol and triglycerides being exposed to spark discharge plasma radiation.
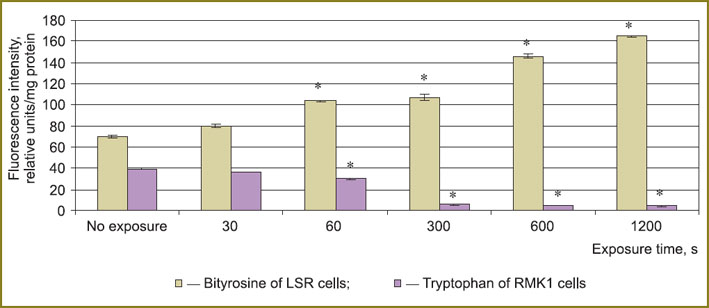
The study of oxidative modification of protein molecules showed the level of bityrosine and glycated proteins to increase by 2.4 and 2.3 times respectively, and tryptophan content to decrease by 7.6 times in LSR cells under plasma radiation by 1200 s of exposure (Fig. 4).
|
Fig. 4. Bityrosine and tryptophan fluorescence intensity of tumor cells after plasma radiation exposure; * — statistically significant in relation to an untreated series, p<0.05 |
In RMK1 cells the growth of bityrosine and glycated proteins was less marked — by 15 and 43% respectively, and tryptophan level decreased by 9 times by 1200 s of exposure (See Fig. 4).
Thus, protein molecules of LSR and RMK1 cells undergo a marked oxidative modification under gas-discharge plasma radiation that will certainly result in numerous malfunctions of protein complexes. Enzymatic function is one of the most significant protein functions. Many enzymatic reactions include transfer of electrons or atomic groups from one substrate to another. Such reactions involve coenzymes, which function as intermediate transporters of atoms or functional groups.It is known that pyridine nucleotides are coenzymes of dehydrogenases, flavin nucleotides are coenzymes of dehydtogenses, oxidases and mono-oxygenases [25]. NAD(P)Н+Н+ was found to fluoresce intensively, while NAD(P)+ does not fluoresce, and flavin nucleotides, on the contrary, are easily reduced and after that they lose their fluorescent property [20, 21] .
Reduced NAD(P)Н+Н+ is also known to be necessary for cell growth, differentiation and programmed cell death. The direction of cell death processes towards apoptosis or necrosis significantly depends on intracellular concentration of NAD+ and ATP. NADН+Н+ and ATP level decrease results in necrosis induction [26]. FADН2 is a cofactor of DNA-photolyase replication enzyme involved in “excision” of thymine dimers.
Relying on the study findings it can be argued that the content of reduced NAD(P)Н+Н+ and oxidized FAD+ increases under plasma radiation: for cell types under study — by 1.5–2 and 2–5 times respectively (Table 2). Such changes can result from damaged metabolic pathways in cells and serve as an inductor of apoptotic processes.
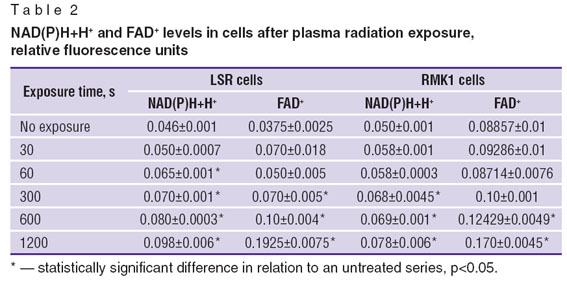 Table 2. NAD(P)Н+Н+ and FAD+ levels in cells after plasma radiation exposure, relative fluorescence units Table 2. NAD(P)Н+Н+ and FAD+ levels in cells after plasma radiation exposure, relative fluorescence units
|
By now it has been found that a nucleotide of prokaryotic cells exposed to plasma radiation degrades [9]. We can assume that plasma radiation can damage DNA of eukaryotic cells as well.
The experiments showed that the number of LSR cells with more than 20% DNA in a tail grows up to 81% by 600th s of exposure (Fig. 5).
Thus, research findings indicate that spark discharge plasma radiation has a destructive effect on DNA molecules of eukaryotic cells that is in agreement with data reported earlier in the studies on bactericidal and sporicidal effect of this radiation [8, 9].
Since in our earlier studies [7] we showed the formation of nitro compounds, in particular, nitric oxide, under plasma radiation in liquid, we might assume that it is they that are actively involved in cytotoxic effect. Nitric oxide, on the one hand, is known to be able to inhibit cell proliferation, and on the other — to induce apoptosis [27, 28]. Cell proliferative activity decrease under nitric oxide can be due to its capability to inactivate iron-containing enzymes involved in the processes of ATP synthesis and DNA molecule replication [29]. It is also known that in the process of free-radical reactions with NO involved, there is possible the formation of high-intensity peroxynitrite oxidant causing covalent alterations in cell protein molecules, DNA damage, and subsequently, apoptosis activation [30, 31]. Thus, it can be concluded that spark discharge plasma radiation has a cytotoxic effect on lymphoid and epithelial LSR and RMK1 tumor cells.
Conclusion. Gas-discharge plasma radiation has a cytotoxic effect on LSR and RMK1 tumor cells, 600-second radiation exposed to LSR and RMK1 cells were found to be a half-lethal dose. Cytotoxic effect is manifested in the changes in cytoplasmic and nuclear membrane structure, in intracellular content, in the increase of cytoplasmic membrane fluidity, in accumulation of oxidative modifications of protein molecules and DNA damage. All changes were in direct relationship to exposure time duration.
Study Funding and Conflict of Interests. The study was not supported by any funds, and the authors have no conflict of interest to disclose.
References
- Kieft I.E., Kurdi M., Stoffels E. Reattachment and apoptosis after plasma-needle treatment of cultures cells. IEEE Trans Plasma Sci 2006; 34(4): 1331–1336, http://dx.doi.org/10.1109/TPS.2006.876511.
- Fridman G., Peddinghaus M., Ayan H., Balasubramanian M., Gutsol A., Brooks A.D., Fridman A., Friedman G. Blood coagulation and living tissue sterilization by floating-electrode dielectric barrier discharge in air. Plasma Chem Plasma Process 2006; 26(4): 425–442, http://dx.doi.org/10.1007/s11090-006-9024-4.
- Laroussi M. Low temperature plasmas for medicine? IEEE Trans Plasma Sci 2009; 37(6): 714–725, http://dx.doi.org/10.1109/TPS.2009.2017267.
- Fridman G., Shereshevsky A., Jost M.M., Brooks A.D., Fridman A., Gutsol A., Vasilets V., Friedman G. Floating electrode dielectric barrier discharge plasma in air promoting apoptotic behavior in melanoma skin cancer cell lines. Plasma Chem Plasma Process 2007; 27(2): 163–176.
- Kim D., Gweon B., Kim D. B., Choe W., Shin J.H. A feasibility study for the cancer therapy using cold plasma. In: 13th International Conference on Biomedical Engineering. Singapore; 2009. Р. 355–357, http://dx.doi.org/10.1007/978-3-540-92841-6_87.
- Kim G., Lee H., Shon C. The effect of a micro plasma on melanoma (G361) cancer cells. Korean Phys Soc 2009; 54: 628–632.
- Ivanova I.P., Trofimova S.V., Karpel Vel Leitner N., Аristova N.А., Arkhipova Е.V., Burkhina О.Е., Sysoeva V.А., Piskaryov I.M. Analiz aktivnykh produktov izlucheniya plazmy iskrovogo razryada, opredelyayushchikh biologicheskie effekty v kletkakh [The analysis of active products of spark discharge plasma radiation determining biological effects in tissues]. Sovrem Tehnol Med — Modern Technologies in Medicine 2012; 2: 20–30.
- Ichetkina A.A., Trofimova S.V., Kryazhev D.V., Ivanova I.P., Smirnov V.F. Vliyanie ul’trafioletovogo izlucheniya i izlucheniya plazmy impul’snogo iskrovogo razryada na zarodyshevye struktury i mitseliy mikromitsetov-destruktorov [The effect of ultraviolet radiation and pulse spark discharge plasma radiation on embryonal structures and mycelium of micromyces-destructors]. Vestnik Nizhegorodskogo gosudarstvennogo universiteta im. N.I. Lobachevskogo — Vestnik of Nizhny Novgorod State University named after N.I. Lobachevsky 2011; 2(2): 196–201.
- Ivanova I.P., Trofimova S.V., Piskaryov I.M., Burkhina О.Е., Sysoeva V.А., Karpel Vel Leitner N. Issledovanie mekhanizmov biotsidnogo deystviya izlucheniya plazmy iskrovogo razryada [The study of biocidal mechanisms of spark discharge plasma radiation]. Sovrem Tehnol Med — Modern Technologies in Medicine 2012; 3: 12–18.
- Chery E. Balkman, Tracy L. Gieger, Marsha M. Zgola, Lionel D. Lewis, Margaret C. McEntee. In vitro characterization of Docetaxel as a radiosensitizer in canine and feline cancer cell lines. Open Journal of Veterinary Medicine 2012; 2: 285–292, http://dx.doi.org/10.4236/ojvm.2012.24045.
- Zhang H.-T., Luo H., Wu J., Lan L.-B., Fan D.-H., Zhu K.-D., Chen X.-Y., Wen M., Liu H.-M. Galangin induces apoptosis of hepatocellular carcinoma cells via the mitochondrial pathway. World J Gastroenterol 2010; 16(27): 3377–3384, http://dx.doi.org/10.3748/wjg.v16.i27.3377.
- Sapozhnikov A.G., Dorosevich A.E. Gistologicheskaya i mikroskopicheskaya tekhnika [Histological and microscopical equipment]. Smolensk: SAU; 2000; 476 p.
- Lutsenko M.T., Ishutina N.A. Sposob otsenki mikrovyazkosti membran eritrotsitov putem vychisleniya koeffitsienta eksimerizatsii pirena KEKS u beremennykh, perenesshikh obostrenie gerpes-virusnoy infektsii v tret’em trimestre gestatsii, s uchetom opredeleniya protsentnogo soderzhaniya oleinovoy kisloty v membranakh eritrotsitov [The assessment method of red blood cell membrane microviscosity by calculating pyrene excimerization coefficient Cexc in pregnant women with recurrent herpes virus infection in the third trimester of gestation based on percentage test of oleic acid in red blood cell membranes]. Patent RF №2467334. МПК G01N33/53. 2012.
- Deryugina A.V., Koryagin A.S., Kopylova S.V., Talamanova M.N. Metody izucheniya stressovykh i adaptatsionnykh reaktsiy organizma po pokazatelyam sistemy krovi [The methods to study stress and adaptive responses of the body by blood system indices]. Nizhny Novgorod: Izdatel’stvo Nizhegorodskogo gosuniversiteta; 2010; 25 p.
- Wheatley R.A. Some recent trends in the analytical chemistry of lipid peroxidation. Тrends in Analytical Chemistry 2000; 19: 617–628.
- Ivanova I.P., Piskarev I.M., Trofimova S.V. Initial stage of lipid peroxidation with HO2 radicals. Kinetic study. American Journal of Physical Chemistry 2013; 2(2): 44–51, http://dx.doi.org/10.11648/j.ajpc.20130202.13.
- Zhang X., Dudek E.J., Liu B., Ding L., Fernandes A.F., Liang J.J., Horwitz J., Taylor A., Shang F. Degradation of C-terminal Truncated αA-crystallins by the ubiquitin-proteasome pathway. Invest Ophthalmol Vis Sci 2007; 48: 4200–4208, http://dx.doi.org/10.1167/iovs.07-0196.
- Dubinina E.E., Gavrovskaya S.V., Kuz’mich E.V., et al. Okislitel’naya modifikatsiya belkov: okislenie triptofana i obrazovanie v ochishchennykh belkakh s ispol’zovaniem sistemy Fentona [Oxidative protein modification: tryptophan oxidation and formation in purified proteins using Fenton reaction]. Biokhimiya — Biochemistry 2002; 67: 413–421.
- Muthenna P., Akileshwari C., Saraswat M., Reddy G.B. Inhibition of advanced glycation end-product formation on eye lens protein by rutin. British Journal of Nutrition 2012 Apr; 107(7): 941–949. Epub 2011 Aug 25, http://dx.doi.org/10.1017/S0007114511004077.
- Farabegoli G., Hellinga C., Heijnen J.J., van Loosdrecht M.C. Study on the use of NADH fluorescence measurements for monitoring waste water treatment systems. Water Res 2003 Jun; 37(11): 2732–2738.
- Zherdeva V.V., Savitskiy A.P. Primenenie lantanidnogo induktivno-rezonansnogo perenosa energii pri izuchenii biologicheskikh protsessov in vitro i in vivo [The use of lanthanide energy transfer by inductive resonance when studying biological processes in vitro and in vivo]. Uspekhi biologicheskoy khimii — Advance of Biological Chemistry 2012; 52: 315–362.
- Durnev A.D., Zhanataev A.K., Anisina E.A., Sidneva E.S., Nikitina V.A., Oganesyants L.A., Seredenin S.B., Bekish V.Ya., Chernukha I.M. Primenenie metoda shchelochnogo gel’-elektroforeza izolirovannykh kletok dlya otsenki genotoksicheskikh svoystv prirodnykh i sinteticheskikh soedineniy [The application of the test of alkaline gel-electrophoresis of isolated cells to assess genotoxic properties of natural and synthetic compounds]. Moscow; 2006; 28 p.
- Boldyrev A.A., Kyayvyaryaynen E.I., Ilyukha V.A. Biomembranologiya [Biomembranology]. Petrozavodsk: Kar NTs RAN; 2006; 226 p.
- Ivanova I.P., Trofimova S.V., Piskaryov I.M., Ichetkina A.A., Burkhina О.Е., Sysoeva V.А. Vliyanie izlucheniya plazmy iskrovogo razryada na modifikatsiyu belkov i lipidov [The influence of the spark discharge plasma radiation on protein’s and lipid’s modification]. Fundamentalnie issledovania — Fundamental Research 2013; 1(3): 525–575.
- Severin E.S. Biokhimiya [Biochemistry]. Moscow: GEOTAR-MED; 2004; 779 p.
- Xia W., Wang Z., Wang Q., Han J., Zhao C., Hong Y., Zeng L., Tang L., Ying W. Roles of NAD / NADH and NADP+ / NADPH in cell death. Current Pharmaceutical Design 2009; 15(1): 12–19, http://dx.doi.org/10.2174/138161209787185832.
- Men’shikova E.B., Zenkov N.K., Reutov V.P. Oksid azota i NO sintazy pri razlichnykh funktsional’nykh sostoyaniyakh [Nitric oxide and NO synthase in different functional conditions]. Biokhimiya — Biochemistry 2000; 65(4): 485–503.
- Choi B.-M., Рае H.-O., Jang S.I. Nitric oxide as a pro-apoptotic as well as anti-apoptotic modulator. Journal of Biochemistry and Molecular Biology 2002; 35(1): 116–126.
- Vanin A.F. Oksid azota v biomeditsinskikh issledovaniyakh [Nitric oxide in biomedical researches]. Vestnik RAMN — Herald of the Academy of Sciences 2000; 4: 3–5.
- Starodubtseva M.N. Peroksinitrit v fiziologii i patologii kletok krovi [Peroxynitrite in physiology and pathology of blood cells]. Moscow: Knizhnyy dom «LIBROKOM»; 2011; 200 p.
- Kwak J.Y., Han M.K., Choi K.S., Park I.H., Park S.Y., Sohn M.H., et al. Cytokines secreted by lymphokine-activated killer cells induce endogenous nitric oxide synthesis and apoptosis in DLD-1 colon cancer cells. Cell Immunol 2000; 203(2): 84–94.