The Role of Mechanical Compression in Human Skin Imaging Using Cross-Polarization Optical Coherence Tomography
Management of biotissue optical properties using cross-polarization optical coherence tomography (CP OCT) enables to acquire additional data on objects under study and can be achieved by applying mechanical compression due to different mechanical and elastic properties of various biotissue layers.
The aim of the investigation was to study the effect of mechanical compression on the formation of in vivo human thin skin CP OCT-images acquired in registered parallel and orthogonal polarizations in relation to initial polarization.
Materials and Methods. In vivo human thin skin was chosen as test object. A series of experiments was carried out to study the effect of skin compression caused by OCT-probe end pressure on contrast of CP OCT-images acquired in parallel and orthogonal polarizations. A group consisted of 7 male volunteers aged 20–50 years with normal skin type, with no pathological changes.
The experiment was performed using an optical coherence tomograph developed by Institute of Applied Physics of the Russian Academy of Sciences (Nizhny Novgorod). Central wavelength of probing radiation was 910 nm, longitudinal resolution — 20 µm, transverse resolution — 25 µm. Probe design enabled to control the force on biotissue at compression using a conjugate dynamometer. A probe diameter was 2.7 mm.
Results. The experiments showed the boundary contrast between layers on CP OCT-images of skin to increase from 3 to 12 dB in parallel and orthogonal polarizations, and from 5 to 12 dB — in orthogonal polarization. Contrast difference at reference time might be related to linear polarization of probing radiation, due to which epidermal and dermal signals are poorly depolarized in relation to initial radiation. In compression, there is a concentration increase of scattering centers in derma resulting in the enhancement of this layer signal and boundary contrast increase, as well as probing radiation depolarization increase due to scattering in this layer, which, in its turn, leads to equalization of contrast values on CP OCT-images in parallel and orthogonal polarizations.
The findings should be taken into consideration when developing the techniques of OCT-diagnosis and interpretation of diagnostic images.
Optical coherence tomography (OCT) technique based on low coherence interferometry is a dynamically developing technique for noninvasive diagnosis of biological tissues. This method enables physicians to image internal structure of the biotissue under study to the depths up to 2 mm with a resolution down to tens of micrometers by registering radiation scattered back by biotissue optical inhomogeneities. Probing of the object is performed using near IR.
In the cross-polarization modification of OCT (CP OCT) [1], employed in this work probing is performed by linearly polarized radiation while registered scattered radiation is divided into two components, which polarizations are parallel and orthogonal to the polarization of the probing radiation (initial polarization). Such signal registration allows us to obtain additional information on the structures of the investigated tissues, for example, on presence of ordered or birefringence structures.
Control of the biotissue optical properties makes it possible to obtain additional information about investigated objects and may be implemented with the help of contrasting [2, 3] or clearing agents [4–12], by changing biotissue temperature conditions [13–18], or by its mechanical compression [19–24]. In the studies of the effect of mechanical compression on the biotissue optical properties, the pressures from 0.1 to 2 MPa are commonly used [25–29], with a painful effect corresponding to pressures above 1.1 MPa [26, 27].
From papers by different research groups [20–24] , it is known that compression can essentially change optical (mainly scattering) properties of the biological tissues being examined. When soft tissues are compressed, intercellular fluid is forced out from the region acted upon, which results in the increase of concentration of scattering centers. The increase of concentration, in its turn, raises the level of the back-scattering signal and depolarization of the probing radiation, and, correspondingly, brightness of the layer on CP OCT-images, obtained in parallel and orthogonal polarization. However, when concentration of the scatterers is essentially high, a reverse effect connected with a dense package of the scatterers, may be observed. Another mechanism of changes in the biotissue optical properties under compression is the reduction of blood flow in the region of action, that leads to the decrease of absorption coefficient and enhancement of OCT-signal in the corresponding area. Compression also results in reduction of the thickness of the object under study, which allows to observe the layers of biotissue at greater depth.
Study of the compression effect on optical properties of biotissues was conducted ex vivo and reported in the work [20]. Samples of human skin, aortas, scleras of the cattle and pigs were used for examination. In the majority of cases decrease of diffuse reflection (a fraction of probing radiation reflected at various angles to the rear half-space ) of the sample under compression was observed, while diffuse transmission (a fraction of probing radiation, passed to the front half-space) and the reduced scattering coefficient μS(1–g) (μS is scattering coefficient of object, and g is the mean cosine of scattering angle) grew.
The work [21] is devoted to revealing possibilities of increasing the optical radiation probing depth in different biological media. Propagation of radiation through turbid media was studied experimentally for layers of the model scattering media and human biotissues, the thickness of which significantly exceeds the free path of photon in the media. A sharp rise of the sample diffuse transmission occurred when a soft scattering tissue was compressed. A relaxation effect of biotissue optical clearing was also noted: after compression the clearing effect did not disappear immediately, but the compressed area remained cleared for 1–3 s.
In papers [22, 23] the depth of OCT visualization was used as a criterion of biotissue clearing degree. In these studies an OCT-system, enabling to obtain OCT-images without a direct contact of the probe with a sample, was used. A grid composed of the optically transparent needles was applied for biotissue compression. The grid was hermetically pressed to the examined sample, followed by further compression of the sample with a vacuum pump. Comparison of visualization depth of the biotissue areas under the “needles” and under a free space showed the rise of the visualization depth in case of biotissue compression. The assessment of changes in relationship of the biotissue refraction index and water content index in time course from the start of action was also given. The experiments were conducted for one volunteer and several samples ex vivo.
In previous studies the efficiency of the biotissue mechanical compression was investigated in order to improve differentiation of the pathological changes in the mucous membrane structures in diagnosing by traditional OCT, where selection of the registered radiation by polarizations is absent [24]. Experiments were designed to study the effect of the compression on the images of rectum ex vivo in case of inflammation and carcinoma, obtained by OCT technique. It was shown that compression in some cases helps to differentiate these pathologic changes. The work also describes the results of numeric simulations of the OCT images of rectum area with inflammation at different compression degrees by Monte-Carlo method. The results of simulations qualitatively agree with the experimental ones.
Later the authors presented the pilot results of their study of the influence of mechanical compression on the formation of OCT-images of thin human skin [30]. Volunteers of three different ages (23, 29, 49 years old) participated in this study, and strong (0.35 MPa) and weak (0.07 MPa) compression was applied. It was estimated that during 3–5 min after the beginning of the exposure increase of contrast by 8–10 dB was observed. It was shown, that there was no substantial difference between the obtained time dependences of contrast for different ages, and this difference becomes less with the increase of compression force. However, these studies considered only a parallel component of the backscattered radiation, whereas its orthogonal component is also informative.
The aim of the investigation is to compare the results of studying the effect of mechanical compression on the formation of thin human skin image in vivo in the parallel and orthogonal channels of the cross-polarization OCT-system.
Materials and Methods
The object of investigation. Dermatology is one of the main fields of OCT application in the diagnostic studies nowadays. It may be explained by the accessibility of the skin and sufficient depth of OCT visualization. However, when contact fiber-optic OCT-probes are employed, it should be taken into account that their interaction with the examined biotissue may affect formation of the image.
A thin human skin in vivo has been chosen as an object of investigation in this work. A series of experiments on the study of the effect of skin compression, caused by OCT-probe end pressure, on the contrast of OCT-images involved a group of 7 male volunteers aged from 20 to 50 years with a normal type of the skin without pathological changes.
The investigation complied with the Declaration of Helsinki (thy Declaration was passed in June, 1984 (Helsinki, Finland) and revised in October, 2000 (Edinburgh, Scotland), and was performed following approval by the Ethics committee of Nizhny Novgorod State Medical Academy. Informed consent was obtained from every patient.
Methods of investigation. The experiment was made using a unique cross-polarization optical coherence tomography developed by the Institute of Applied Physics of the Russian Academy of Sciencies [31, 32]. Probing is performed by linearly polarized radiation. The central wavelength is 910 nm, spectral width — 50 nm, longitudinal resolution — 20 μm, transverse resolution — 25 μm. The registered CP OCT-signal was divided into two channels, in which radiation, backscattered from the object, with polarizations parallel and orthogonal to the initial polarization of the probing radiation was registered. The design of the fiber-optic probe allows one to control the force of pressure on the biotissue during compression with the help of the coupled dynamometer. The probe diameter was 2.7 mm.
The CP OCT-probe coupled with the dynamometer measuring the force of pressing the probe to the sample, was placed perpendicularly to the sample surface with the force of 0.35±0.04 MPa (Fig. 1). The compression force was chosen to provide full contact of the probe with the skin and cause insignificant discomfort. Such compression is a noninvasive action, all changes are reversible, and their consequences resolve during 20 min after the exposure stops. Further increase in the pressure force may cause hematoma, which may be classified as an invasive action [24].
 Fig. 1. CP OCT-monitoring of the skin area compression Fig. 1. CP OCT-monitoring of the skin area compression
|
A skin area underwent continuous CP OCT-monitoring for 7 min (CP OCT-images were obtained with the frequency of 0.2 Hz).The experiment was conducted at the room temperature (20°C). During the process of measurement a force pressing the CP OCT-probe to the biotissue surface was maintained at a constant level for the whole time of observation.
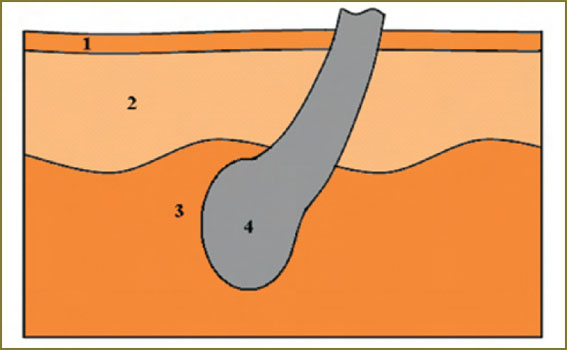 Fig. 2. Schematic picture of the human skin with structural elements, distinguishable on CP OCT-images: 1 — stratum corneum, 2 — cellular layers of epidermis, 3 — dermis, 4 — hair follicle Fig. 2. Schematic picture of the human skin with structural elements, distinguishable on CP OCT-images: 1 — stratum corneum, 2 — cellular layers of epidermis, 3 — dermis, 4 — hair follicle
|
After CP OCT-monitoring, the images obtained were digitally processed, and according to this data time dependence of the contrast of the border between the epidermal and dermal layers (further–contrast) starting from the beginning of exposure was determined. Images, obtained in the parallel and orthogonal polarizations were considered separately.
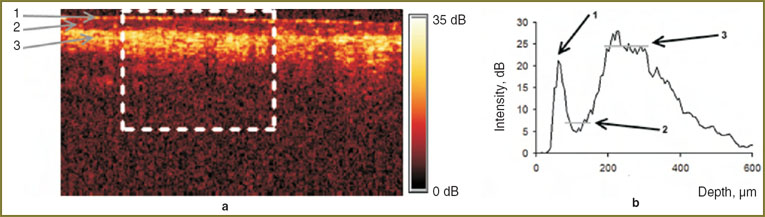
Methods of determining contrast on OCT-images. A term of structural element contrast (layer borders — in this study) in the OCT-image was used as a qualitative characteristic of the effect of compression on CP OCT images. A homogenous area was selected along the transverse coordinate in the CP OCT image (Fig. 3, a) and the OCT-signal was averaged within the selected area along the transverse coordinate. The relation of CP OCT-signal intensity to the depth (averaged A-scan) obtained as a result of averaging was used to determine the intensity of OCT-signal from the examined layers, designated in decibels (Fig. 3, b). The difference between signal intensities corresponds to the relation of these intensities, designated in decibels.
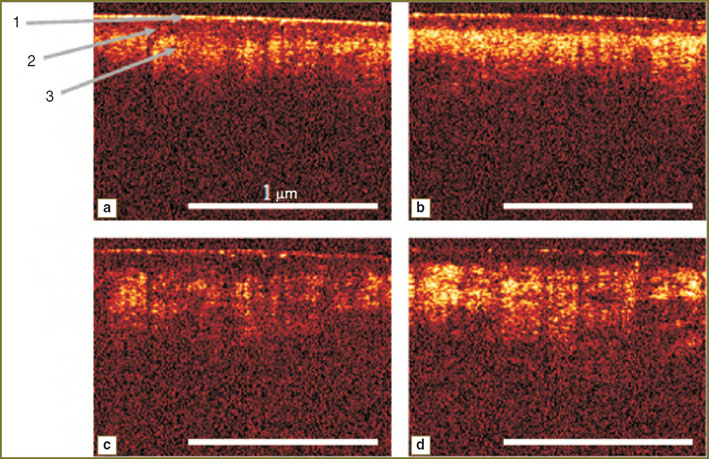
Results and Discussion. OCT-images of a thin human skin have a stratified arrangement corresponding to the anatomical skin structure: corneous layer, cellular layers of epidermis, papillary and reticular dermis. Brighter regions correspond to strongly scattering layers, while darker areas are related to the weakly scattering or strongly absorbing layers or structural elements, such as, for example, the ducts of sebaceous or sweat glands, capillaries, hair follicles. As there were no significant changes on CP OCT-images from 5 to 7th minute after the beginning of exposure, typical CP OCT-images of the skin, obtained in the parallel and orthogonal polarizations immediately after and in 5 min after its beginning (Fig. 4), were selected.
Averaged A-scans for parallel and orthogonal polarization (Fig. 5) show that OCT-signal from the dermis in 5 min after the beginning of exposure is higher both in parallel and orthogonal channels. At the same time, a signal from the cellular layers of epidermis did not change significantly. Thus, mechanical compression enables to increase the border contrast of these layers.
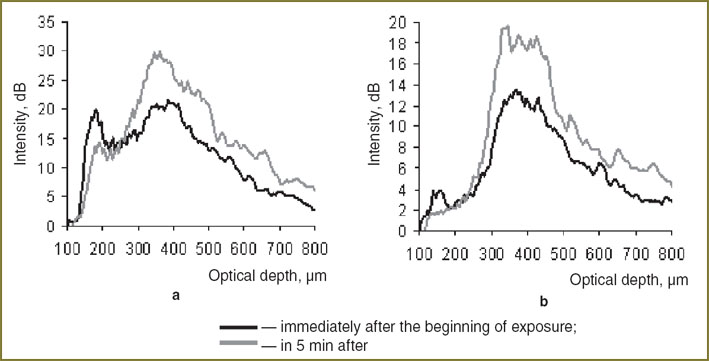 Fig. 5. Averaged A-scans of CP OCT-image immediately after the exposure and in 5 min after its beginning in parallel (a) and orthogonal (b) channels Fig. 5. Averaged A-scans of CP OCT-image immediately after the exposure and in 5 min after its beginning in parallel (a) and orthogonal (b) channels
|
The presented images illustrate, that in 5 min after the beginning of exposure a significant enhancement of CP OCT-signal from the dermis occurs, which results in the growth of the border contrast of this layer in CP OCT-images, obtained in parallel as well as in orthogonal polarizations. This effect is likely to be connected with displacement of intracellular fluid from the area of action [24]. Reduction of capillary bloodstream can also influence the effect, which leads to the decrease of the local coefficient of absorption, and, consequently to the rise of OCT-signal from this area. Contrast difference on CP OCT-images, obtained in parallel and orthogonal polarizations is connected with the fact that linearly-polarized probing radiation is weakly depolarized in epidermis, therefore backscattering from epidermis and dermis contributes mainly to the image in the parallel channel. In dermis, in its turn, strong depolarization of the signal takes place, making the levels of CP OCT-signals from these layers in the parallel and orthogonal polarization comparable. In compression increase of scatterer concentration occurs, which causes stronger depolarization of the probing radiation, and, therefore, equalization of the contrast value in both polarizations.
After data analysis on CP OCT-images, the value of contrast was calculated (using the described methodology) at each moment of the time, then the values were averaged for all volunteers. The obtained time dependencies of contrast starting from the beginning of compression for parallel and orthogonal polarizations (Fig. 6) confirm the suggestions, made previously, on the influence of compression on the degree of depolarization of the probing radiation. Both dependencies are of the monotonous growing character. The contrast in parallel polarization at the starting moment of time exceeds that in the orthogonal one, however, the contrast values become equal with time (in parallel polarization it grows from 3 to 12 dB, while in the orthogonal one — from 5 to 12 dB). Thus, in the long-term compression the level of the signal in orthogonal polarization is comparable with the signal level in the parallel polarization. The difference of contrasts at the starting moment of time is connected with the fact, that because of the weak signal depolarization in epidermis, the CP OCT-signal, received from it, is polarized chiefly parallel to the initial polarization of the probing radiation. The basic radiation depolarization takes place in the dermis, which is caused by greater concentration of scatterers in this layer, as well as by their birefringence properties. An increase of the concentration of scattering centers occurs in the dermis under compression, the signal from the area grows, leading to the enhancement of the border contrast. Besides, depolarization of the probing radiation goes on more effectively, resulting to the leveling of contrast values in parallel and orthogonal polarizations.
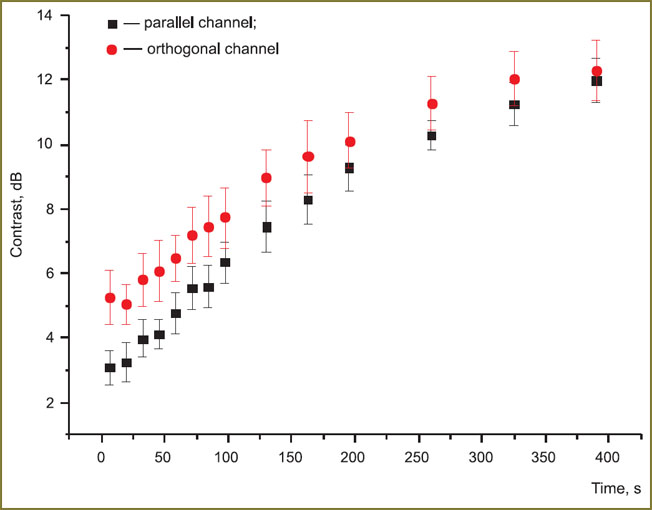 Fig. 6. Contrast to time relations on OCT-images in parallel and orthogonal polarizations. Vertical lines designate error of the mean Fig. 6. Contrast to time relations on OCT-images in parallel and orthogonal polarizations. Vertical lines designate error of the mean
|
Conclusion. We report on the study of the effect of mechanical compression, caused by 0.35±0.04 MPa pressure on the surface of the biotissue during 7 min, on the contrast of the border between the cellular layers of epidermis and papillary dermis in CP OCT images. Probing by linearly-polarized radiation with simultaneous detection of the OCT-signal in parallel and orthogonal reference polarizations was employed. The results indicate that in developing the guidelines of CP OCT diagnosis, the compression technique may be applied for obtaining supplemental information on the structure. However, when contact CP OCT-probes are used, compression of the examined biotissue gives distorted CP OCT-images and changes contrast and absolute brightness of the layers in both polarizations, which will depend on the pressure force and time of measurement. In this connection, while investigating dynamic processes in biotissues by CP OCT technique using contact probes, it is necessary to perform a control measurement similar in duration and acting force to the basic one, which may serve as a reference when analyzing the results.
Study Funding. The work was supported by Russian Foundation for Basic Research (projects 10-02-00744a, 12-02-31191) and Ministry of Education and Science of the Russian Federation, agreements 8722 and 8147.
References
- Gladkova N.D., Streltsova O.S., Zagaynova E.V., Kiseleva E.B., Gelikonov V.M., Gelikonov G.V., Karabut M.M., Yunusova K.E., Evdokimova O.S. Cross-polarization optical coherence tomography for early bladder-cancer detection: statistical study. J Biophotonics 2011; 4(7–8): 519–532, http://dx.doi.org/10.1002/jbio.201000088.
- Zagaynova E.V., Shirmanova M.V., Kirillin M.Yu., Khlebtsov B.N., Orlova A.G., Balalaeva I.V., Sirotkina M.A., Bugrova M.L., Agrba P.D., Kamensky V.A. Contrasting properties of gold nanoparticles for optical coherence tomography: phantom, in vivo studies and Monte Carlo simulation. Phys Med Biol 2008; 53: 4995, http://dx.doi.org/10.1088/0031-9155/53/18/010.
- Troutman T., Barton J.K., Romanowski M. Optical coherence tomography with plasmon resonant nanorods of gold. Optics Letters 2007; 32(11): 1438–1440, http://dx.doi.org/10.1364/OL.32.001438.
- Tuchin V.V. Opticheskaya biomeditsinskaya diagnostika [Optical biomedical diagnostics]. Vol. 2. Moscow: Fizmatlit; 2007.
- Vargas G., Chan E.K., Barton J.K., Rylander H.G. 3rd., Welch A.J. Use of an agent to reduce scattering in skin. Lasers Surg Med 1999; 24(2): 133–141.
- Veiro J.A., Cumming P.G. Imaging of skin epidermis from various origins using confocal laser microscopy. Dermatology 1994; 89: 16–17.
- Wang R.K., Xu X., Tuchin V.V., Elder J.B. Concurrent enhancement of imaging depth and contrast for optical coherence tomography by hyperosmotic agents. JOSA B 2001 Jul; 18(7): 948–958, http://dx.doi.org/10.1364/JOSAB.18.000948.
- Welzel J., Lankenau E., Birngruber R., Engelhardt R. Optical coherence tomography of the human skin. Journal of the American Academy of Dermatology 1997; 37(6): 958–963.
- Welzel J. Optical coherence tomography in dermatology: a review. Skin Res Technol 2001; 7(1): 1–9, http://dx.doi.org/10.1034/j.1600-0846.2001.007001001.x.
- Welzel J., Lankenau E., Birngruber R., Engelhardt R. Optical coherence tomography of the skin. In: Elsner P., Barel A.O., Berardesca E., Gabard B., Serup J. (eds). Skin bioengineering. Techniques and applications in dermatology and cosmetology. Curr Probl Dermatol. Basel, Karger; 1998; Vol. 26; p. 27–37, http://dx.doi.org/10.1159/000060573.
- Petrova G.A. Vozmozhnosti i mesto opticheskoy kogerentnoy tomografii v diagnostike bolezney kozhi. Dis. … dokt. med. nauk [Optical coherence tomography potential and position in skin disease diagnostics]. Nizhny Novgorod, 2003.
- Petrova G.A., Derpalyuk E.N., Gladkova N.D., Nikulin N.K., Iksanov R.R., Gelikonov G.V., Donchenko E.A. Puti uvelicheniya informativnosti opticheskoy kogerentnoy tomografii v dermatokosmetologii [Informativity improvement methods of optical coherence tomography in dermatology and cosmetology]. Eksperimental’naya i klinicheskaya dermatokosmetologiya — Experimental and clinical dermatology and cosmetology 2005; 3(10): 7.
- Bashkatov A.N., Genina E.A. Water refractive index in dependence on temperature and wavelength: a simple approximation. Proc SPIE 2003; 5068: 393, http://dx.doi.org/10.1117/12.518857.
- Martinsen P., Charlier J.-L., Willcox T., Warman G., McGlone A., K?nnemeyer R. Temperature dependence of near-infrared spectra of whole blood. J Biomed Opt 2008; 13(3): 034016, http://dx.doi.org/10.1117/1.2943191.
- Khalil O.S., Yeh S., Lowery M.G., Wu X., Hanna C.F., Kantor S., Jeng T.-W., Kanger J.S., Bolt R.A., de Mul F.F. Temperature modulation of the visible and near infrared absorption and scattering coefficients of human skin. J Biomed Opt 2003; 8(2): 191–205, http://dx.doi.org/10.1117/1.1559997.
- Ouyang Q., Zhu D., Luo Q., Gong H., Luo Q. Modulation of temperature on optical properties of rat skin in vivo. Proc SPIE 2007; 6534: 65343I, http://dx.doi.org/10.1117/12.741499.
- Laufer J., Simpson R., Kohl M., Essenpreis M., Cope M. Effect of temperature on the optical properties of ex vivo human dermis and subdermis. Phys Med Biol 1998; 43(9): 2479–2489, http://dx.doi.org/10.1088/0031-9155/43/9/004.
- van der Meer F.J., Faber D.J., Çilesiz I., van Gemert M.J.C., van Leeuwen T.G. Temperature-dependent optical properties of individual vascular wall components measured by optical coherence tomography. J Biomed Opt 2006; 11(4): 041120, http://dx.doi.org/10.1117/1.2333613.
- Sarvazyan A.P., Skovoroda A.R. Method and apparatus for elasticity imaging. Patent US 5,524,636. 1996.
- Chan E.K., Sorg B., Protsenko D., O’Neil M., Motamedi M., Welch A.J. Effects of compression on soft tissue optical properties. IEEE J Selected Topics in Quantum Electronics 1996; 2(4): 943–950, http://dx.doi.org/10.1109/2944.577320.
- Askaryan G.A. Uvelichenie prokhozhdeniya lazernogo i drugogo izlucheniya cherez myagkie mutnye fizicheskie i biologicheskie sredy [Enhancement of laser and other radiation penetration through soft turbid physical and biological media]. Kvantovaya elektronika — Quantum electronics 1982; 9(7): 1370–1383.
- Rylander C.G., Milner T.E., Baranov S.A., Nelson J.S. Mechanical tissue optical clearing devices: enhancement of light penetration and heating of ex vivo porcine skin and adipose tissue. Lasers Surg Med 2008; 40(10): 688–694, http://dx.doi.org/10.1002/lsm.20718.
- Drew C., Milner T.E., Rylander C.G. Mechanical tissue optical clearing devices: evaluation of enhanced light penetration in skin using optical coherence tomography. J Biomed Opt 2009; 14(6): 064019, http://dx.doi.org/ 10.1117/1.3268441.
- Agrba P.D., Kirillin M.Yu., Abelevich A.I., Zagaynova E.V., Kamenskiy V.A. Kompressiya kak metod povysheniya informativnosti opticheskoy kogerentnoy tomografii biotkaney [Compression as informativity improvement method of biotissue optical coherence tomography]. Optika i spektroskopiya — Optics and Spectroscopy 2009; 107(6): 901–906.
- Izquierdo-Romа A., Vogt W.C., Hyacinth L., Rylander C.G. Mechanical tissue optical clearing technique increases imaging resolution and contrast through ex vivo porcine skin. Lasers in Surgery and Medicine 2011; 43(8): 814–823, http://dx.doi.org/10.1002/lsm.21105.
- Lee W.C., Zhang M., Mak A.F. Regional differences in pain threshold and tolerance of the transtibial residual limb: including the effects of age and interface material. Archives of Physical Medicine and Rehabilitation 2005; 86(4): 641–649, http://dx.doi.org/10.1016/j.apmr.2004.08.005.
- Xiong S., Goonetilleke R.S., Witana C.P., Rodrigo W.D. An indentation apparatus for evaluating discomfort and pain thresholds in conjunction with mechanical properties of foot tissue in vivo. J Rehabil Res Dev 2010; 47(7): 629–641, http://dx.doi.org/10.1682/JRRD.2009.09.0152.
- Fischer A.A. Pressure tolerance over muscles and bones in normal subjects. Arch Phys Med Rehabil 1986; 67(6): 406–409.
- Pickering G., Jourdan D., Eschalier A., Dubray C. Impact of age, gender and cognitive functioning on pain perception. Gerontology 2002; 48(2): 112–118, http://dx.doi.org/10.1159/000048937.
- Kirillin M.Yu., Agrba P.D., Kamensky V.A. In vivo study of the effect of mechanical compression on formation of OCT images of human skin. J Biophotonics 2010; 3(12): 752–758, http://dx.doi.org/10.1002/jbio.201000063.
- Kuranov R.V., Sapozhnikova V.V., Turchin I.V., Zagainova E.V., Gelikonov V.M., Kamensky V.A., et al. Complementary use of cross-polarization and standard OCT for differential diagnosis of pathological tissues. Optics Express 2002; 10(15): 707–713, http://dx.doi.org/10.1364/OE.10.000707.
- Feldchtein F.I., Gelikonov V.M., Gelikonov G.V. Polarization-sensitive common path optical coherence reflectometry/tomography device. Patent US 7728985 B2. 2010.