Modern Technologies of Open-Angle Glaucoma Surgery
Currently, glaucoma surgery has become a major technique. Surgery is a method of choice in open-angle glaucoma, especially in those cases when conservative and laser treatments have no effect, as well as if glaucoma is inaccessible or a patient has low treatment compliance. In this review the authors give the information about the development of main glaucoma surgery directions, such as removal of pupillary block, the anterior chamber fistulization, the reduction of intraocular fluid production. Drainage surgery, its history and modern technical devices have been characterized in detail. The design of drainage devices has been improved towards their size reduction, extension of filtration area and the development of valve mechanisms. Indications for their implementation in open-angle glaucoma treatment, and the factors contributing to a successful treatment have been discussed. There have described early and late complications of microdrainage and paid particular attention to obliteration of developed outflow pathways, being the main problem as they reduce the effect of filtering and draining operations. The authors have presented a number of advanced micro-invasive technologies, both used in clinical practice (Ex-PRESS™ mini-shunt, Trabectome™, iStent, canaloplasty and viscocanalostomy), and those being under clinical study — SOLX Gold Micro-Shunt, CyPass, Hydrus™ Microstent a canalicular scaffold, AqueSys Microfistula Implant. There has been presented the information on design and technology of these devices with their detailed classification based on the differences in their mechanism of action, operative approach type and material used.
In accordance with current concepts, glaucoma is a group of diseases characterized by increased intraocular pressure (IOP), which is higher than tolerance limit, accompanied by optic neuropathy and typical decrease of visual functions. Glaucoma management primarily aims at maintaining visual organ function. Glaucoma therapy is based on the disease monitoring in order to prevent progressive glaucoma optic neuropathy, stabilization of visual functions by maintaining target IOP. IOP decrease by every 1 mm Hg reduces glaucoma progression risk by 10% [1], and pressure reduction should be stable, since tunneling of vision relates more to pressure variation and, statistically, to standard error, rather than to IOP arithmetic mean [2, 3]. And IOP fluctuations induced by various factors can reach high values [4].
Currently, glaucoma surgery has become a major technique, and in some cases it is a method of choice, even if glaucoma is newly diagnosed. The experience of Nizhny Novgorod Regional Ophthalmological Clinic (Russia) shows that more than half the patients who sought medical advice for glaucoma in 2012 required surgical management. There are various approaches to determine surgical indications for glaucoma patients. According to meta-analysis carried out by J. Burr et al., there are no significant differences between visual field variation degree within five years in groups of patients who primarily underwent conservative or surgical treatment [5]. Surgery is a method of choice in open angle glaucoma, if conservative and laser treatment has no effect, as well as in case of inaccessibility or low patient’s compliance. The last-mentioned should be paid particular attention to taking into consideration the represented data on greater significance of IOP fluctuation rather than IOP mean value [6].
Background. In 1856 Albrecht von Graefe (1828–1870) for the first time performed iridectomy in acute glaucoma attack, and shortly after that de Wecker performed the first sclerectomy (1858), in Russia the first fistulizing operation — oblique sclerectomy sclerotomy — was carried out by А.N. Maklakov (1887). Subsequently, cyclodialysis (1900) and thermocauterization (1932) were suggested. Thus, by the early 1930s there were formed such basic glaucoma operative approaches as papillary block removal, anterior chamber fistulization, intraocular fluid production decrease [7]. Trabeculectomy was developed in experiment by H.S. Sugar (1961), and introduced in clinical practice by J.E. Cairns (1968). Subsequently, antimetabolites — 5-fluorouracil and Mitomycin-C were used in glaucoma surgery. Deep scleroctomy was suggested by S.N. Fedorov (1974), non-penetrating sclerectomy — by V.I. Kozlov et al. (1987), sinustrabeculectomy and sinusotomy — by М.М. Krasnov et al [8–10]. However, insufficient efficacy of the proposed techniques, a great number of refractory glaucoma patients characterized by persistent increased IOP, resistant to medical and surgical intervention have caused the search and development of new techniques and devices for glaucoma surgical management.
Drainage devices. Drainage surgery developed in incremental steps [11]. Setons (Lat. seta — bristle) draining aqueous humor along the proper surface as outflow drainage (silk thread, artery wall, polymer materials, etc) was first used as early as in 1866 by Wecker (a gold wire). Later, there were brought forward shunt-tubes draining anterior chamber humor in a filtering subconjunctival bleb. Further, shunt construction was complicated, drainage devices were developed, in which a distal end of a shunt-tube was connected to polymer case (body) of a drain tube fixed posteriorly from (scleral sulcus) limbus. Drainage devices were elaborated towards size reduction, filtration area increase and development of valve mechanisms.
Currently, among drainage devices, the most relevant ones in clinical practice are nonvalved drainage devices Molteno (Molteno Ophthalmic Ltd., New Zealand) and Baerveldt (Advanced Medical Optics, Inc., USA), and valve models Krupin (Eagle Vision, Inc., USA) and Ahmed (New World Medical, Inc., USA) [12, 13]. Their application enables to have an effect similar to that of conventional surgeries, however, there is no convincing evidence confirming the advantage of a particular device [14]. The main indication for drainage device usage is refractory glaucoma, which includes such clinical forms as a failed operated primary glaucoma, neovascular glaucoma, uveal glaucoma, pigmentary glaucoma, juvenile glaucoma, closed-angle “creeping” glaucoma, etc, but today micro-shunting is widely used also in patients with primary open-angle glaucoma at the first stage of surgical treatment [15].
Ahmed implant due to its valve mechanism is characterized by the higher safety level in relation to postoperative hypotony. A valve mechanism has a cone-shaped chamber due to Venturi effect, valve opening pressure being 8.0 mm Hg. By its clinical efficiency the device is as good as Mitomycin-С-based trabeculectomy [16]. In particular, its efficiency in refractory glaucoma 1 or 5 years after the surgery (IOP maintenance in the range from 5 to 21 mm Hg, with or without medicinal drugs) is 80 and 49% respectively [17]. However, year by year after implantation, the number of working devices of any type decreases, first of all, due to incapsulation, and 5 years later the number averages 50% [12, 16, 17].
Obliteration of developed outflow pathways is a major problem eventually reducing the effect of all filtering and draining operations using both common and novel devices. Doctor D.Y. Yu et al. [18] was the first who focused ophthalmologists’ attention on the fact that correct formation of outflow pathway is of great importance as well as device construction. Operative technique should primarily provide minimum conjunctiva damage, and, secondarily, prepare outflow pathway to its lymph net. W. Schmidt et al. [19] thinks the solution is in the differentiated pharmacological accompanying of surgeries, in particular, in the selection of anti-metabolites depending on proliferation properties and differentiation of fibroblast subtypes forming corresponding eye structures.
However, drainage micro-surgery has its drawbacks, both in operative technique, and in design and size of devices, biocompatibility of materials [20] that in some cases result in early and late complications related mainly to failure of developed outflow pathways (See the Table). Moreover, if draining devices are placed improperly there can develop corneal edema, keratitis, cataract.
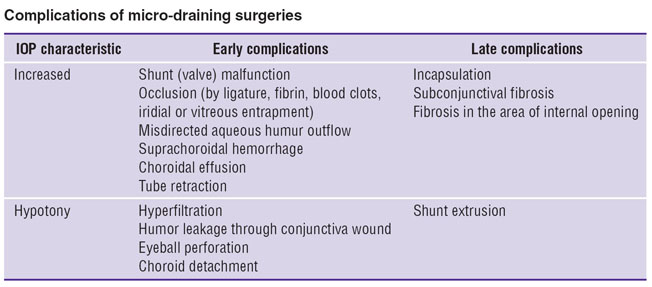 Complications of micro-draining surgeries Complications of micro-draining surgeries
|
New-generation micro-invasive technologies. In the late XX century new-generation micro-invasive technologies for glaucoma management were developed. Among them there are the following devices and surgical treatment modalities [17, 21, 22]:
1) Ex-PRESS™ mini-shunt (Alcon, USA);
2) Trabectome™ (NeoMedix, Inc., USA);
3) iStent (Glaukos Corporation, USA);
4) Canaloplasty (iScience Interventional, USA);
5) SOLX Gold Micro-Shunt (SOLX, Inc., USA);
6) CyPass (Transcend Medical, USA);
7) Hydrus™ Microstent a canalicular scaffold (Ivantis Inc., USA);
8) AqueSys Microfistula Implant (AqueSys, Inc., USA).
New techniques differ in the mechanism of hypotensive effect, material of devices, as well as in operative technologies. Exposure of anterior chamber angle structures by conjunctival and scleral flap dissection has been called external approach — ab externo, and penetration into anterior chamber through corneal incision (like in phacoemulsification) — internal approach, аb interno.
It should be noted, that not all the mentioned techniques are used in practice. Food and Drug Administration (FDA), USA, has not yet approved SOLX Gold Micro-Shunt, CyPass, Hydrus™ Microstent, AqueSys Microfistula Implant. Trabecular micro-stent iStent combined with cataract surgery in 2012 was recommended for application.
Ex-PRESS™ (Excessive Pressure Regulation Shunt System) mini-shunt was suggested in 1998 (M. Belkin, Y. Glovinsky), made in Israel (Optonol Ltd., since 2010 — Alcon). A shunt is made of medical steel, it is a tube, 2.64 mm in length, outside diameter being 400 µm (27 G) and inside diameter — 50 µm. The device has a spur-like projection for fixation in anterior chamber, plate on base, and extra anti-lock opening situated on a semi-axis so that if a shunt is placed in anterior chamber, it will face cornea. Drainage device is implanted in anterior chamber under scleral flap through an opening in scleral sulcus area (ab externo) followed by scleral flap and conjunctiva suturing. Implantation characteristics have been described by a number of foreign and Russian researchers [23, 24], the description of shunt explantation technique and re-implantation in case of intraoperative error was novel [25, 26]. There has been presented shunt implantation technique through sclera tunnel without conjunctiva dissection [27]. The technique complies with the abovementioned requirements for minimal conjunctiva damage.
Minimally invasive implantation, a low level of intra- and postoperative complications, capability to lead to a sustained IOP decrease enable to compare mini-shunt efficiency with that of trabeculectomy [28, 29]. A prospective, randomized study [30] involving 15 patients with open-angle glaucoma of both eyes appeared to be the most descriptive and significant. All the patients underwent trabeculectomy on one eye, and implantation — on a fellow eye. A two-year follow-up showed these surgeries to be little different in postoperative IOP, while Ex-PRESS™ implantation less frequently causes complications (20 versus 33%) and the necessity of postoperative surgeries (0 versus 27%), as well as requires the administration of the fewer IOP-lowering agents. Mini-shunt implantation does not result in changes of anterior segment parameters (angle size, depth, volume) within three follow-up months [31]. As for the material (stainless steel) biocompatibility is concerned, animal experiments using Mitomycin-С showed no significant differences in tissue structure in the formation of filtering bleb and capsule around Ex-PRESS™ mini-shunt and a silicon drainage tube [32]. Histological examination (post mortem and after enucleation) of patients operated on for glaucoma with mini-shunt implantation revealed good biocompatibility of the device, formation of a thin fibrous cap, and no inflammatory cells 24 months after the surgery [33, 34]. Considerable experience of this glaucoma therapy technique application in Russia and abroad gives various authors the reason to recommend mini-shunt implantation as a primary surgery in case there are medical indications for glaucoma therapy [15], or as trabeculectomy alternative in a group of patients with target IOP 13–15 mm Hg [23]. A mini-shunt is proved to maintain its position and have no effect on MRI quality, 1.5 and 3.0 T, but comes into motion if magnetic field density is 4.7 T [35, 36]. We consider that a metal shunt located in the anterior segment is prone to tissue cutting. Moreover, any metal device even made of medical steel when placed in reactive media is exposed to an oxidative process.
Ab interno trabeculectomy performed using Trabectome™, and iStent implantation are referred to аb interno surgeries on Schlemm’s canal and gonioscopy-assisted [37]. The procedures aim at overcoming the resistance of trabecular apparatus in open-angle glaucoma and prepare aqueous outflow pathway from anterior segment to Schlemm’s canal avoiding a trabecular net. Ab interno trabeculectomy includes focal ablation and cauterization of a trabecular net over a period from 90 to 120° using Trabectome™, which has a tip — a microelectrocauter [38]. When a micro-stent is placed, direct connection between the anterior segment and Schlemm’s canal is formed. iStent micro-stent is made of medical titanium, it is a heparin-covered, right-angled tube, 1 mm in length, outside diameter being 250 µm and inside diameter — 120 µm. The anterior segment via corneal short-scar incision at 3 o’clock is filled by viscoelastic, a applicator with a stent is placed and put through anterior chamber till scleral spur and iris root are reached in lower nasal quadrant, the stent is placed in Schlemm canal lumen by its pointed end, while the second end remains turned towards anterior segment [39].
Canaloplasty (iScience) and viscocanalostomy are referred to ab externo operations on Schlemm`s canal and combined with filtration surgery [40, 41]. In viscocanalostomy, viscoelastic is injected in Schlemm’s canal in order to broaden canal lumen and form mini-ruptures in its inner wall. In canaloplasty, viscoelastic is injected in Schlemm`s canal through an incision similar to that in trabeculectomy, then a flexible probe with a light emitting diode at the end (iScience, diameter of different types vary from 250 to 400 µm) is inserted full-length in the canal lumen [42]. Polypropylene thread 10-0 is inserted into Schlemm`s canal lumen with the help of a probe, the ends of the thread being tied with tension that provides Schlemm`s canal lumen retention in a long-term postoperative period and more significant, compared to viscocanalostomy, IOP decrease [43]. Other surgeries are used in both open-angle and narrow-angle glaucomas including those performed as one-stage operations with phacoemulsification. The necessary condition of effectiveness is the integrity of the distal part of outflow — collecting canaliculi, episcleral recepient veins; it can be determined by blood reflux provocation in gonioscopy or according to fluorescent canalography. The absence of artificial openings and filtering bleb on eyeball surface, as well as aqueous outflow control determined by physiological resistance of classic outflow pathway should result in complication risk reduction, particularly, hypotony. Indeed, ab interno trabeculectomy and canaloplasty less frequently cause side effects [44–46].
SOLX Gold Micro-Shunt is a gold plate, 3.2×5.2 mm in size, with numerous micro-channels. A shunt is implanted in suprachoroidal space ab externo, where aqueous humor is drained via channels from the anterior chamber under pressure gradient [47].
Micro-shunt CyPass is a perforated tube made of polyamide material (thermoresistant biocompatible polymer), 6.35 mm in length, inside diameter being 0.3 mm, outside — 0.51 mm, at one end there is plate and three retaining rings. Viscoelastic fills the anterior chamber through 1.5 mm corneal dissection, then cyclodialysis is performed using a blunt end of a stent mounted on delivery system. After that a micro-stent is pushed in suprachoroidal space (ab interno). The ring fix the stent in sclera spur area and iris root, plate is left faced towards the anterior chamber. First results of micro-stent application showed its efficiency and safety in open-angle glaucoma management [48].
Hydrus™ Microstent is a tube frame made of nitinol, 8 mm in length, implanted ab interno in Sclemm’s canal lumen. Nitinol is shape memory material, titanium NiTi nickelide, which is not alloy but intermetallide — a compound with fixed atomic ratio. The name of the material is an acronym consisting of the first letters of the components’ elements and the place of its discovery — Naval Ordnance Laboratory, USA (Nickel Titanium Naval Ordnance Laboratory). Currently, Hydrus™ Microstent efficiency is being studied within the framework of international clinical trials “Hydrus IV” (phacoemulsification combined with micro-stent implantation has been performed since February 2012) and experimental studies [49].
AqueSys Microfistula Implant, as well as its new version — XEN Gel Stent, is a gelatin (hydrolyzed collagen) tube. The device is implanted ab interno in suprachoroidal space using injecting device similarly used in phacoemulsification. Gelatin drainage changes pattern in tissues. Now phase 3 of clinical trials is being carried out (2012–2014).
Based on the most essential characteristics of the techniques described, we suggest the following classification of modern surgical techniques for glaucoma management using drainage devices.
I. By mechanism of action:
1. Draining of anterior chamber humor into episcleral reservoire: drainage devices Molteno, Baerveldt, Krupin, Ahmed Glaucoma Valve.
2. Formation of a new outflow pathway through a filtering bleb under scleral or conjunctival flap: Ex-PRESS™ mini-shunt.
3. Formation of a new outflow pathway into suprachoroidal space — intrascleral systems: AqueSys Microfistula Implant, CyPass, Gold Micro-Shunt.
4. Forcing of aqueous outflow in Sclemm`s canal: аb interno trabeculectomy, iStent, Hydrus™ Microstent, canaloplasty (iScience).
II. By operative approach type:
1. External approach (ab externo): canaloplasty (iScience), Gold Micro-Shunt, Ex-PRESS™.
2. Internal approach (аb interno): AqueSys Microfistula Implant, CyPass, iStent, Hydrus™ Microstent.
III. By the material of implantable devices:
1. Metals and alloys: Ex-PRESS™, SOLX Gold Micro-Shunt, Hydrus™ Microstent, iStent.
2. Polymer materials: canaloplasty (iScience), CyPass.
3. Biomolecules: AqueSys Microfistula Implant.
As the experts from evidence-based healthcare center (John Hopkins University, USA) note, now it is impossible yet to conclude about the efficacy of a particular technique for glaucoma management, since researchers are not provided enough information on the changes of optic nerve and visual field in patients under study [50, 51]. Not all the existing techniques can be compared with the gold standard of conventional surgery in terms of hypotensive effect and the rate of complications [52, 53]. New randomized studies are required to prove clinical efficiency of novel technologies compared with conventional ones concerning IOP reduction, as well as steady stabilization of visual functions, the latter being the main goal of glaucoma treatment.
Study Funding and Conflict of Interests. The study was not supported by any funds, and the authors have no conflict of interest to disclose.
References
- Leske M.C., Heijl A., Hussein M., Bengtsson B., Hyman L., Komaroff E. Early Manifest Glaucoma Trial Group. Factors for glaucoma progression and the effect of treatment: the early manifest glaucoma trial. Arch Ophthalmol 2003 Jan; 121(1): 48–56, http://dx.doi.org/10.1001/archopht.121.1.48.
- Caprioli J., Coleman A.L. Intraocular pressure fluctuation: a risk factor for visual field progression at low intraocular pressures in the advanced glaucoma intervention study. Ophthalmology 2008 Jul; 115(7): 1123–1129.e3. Epub 2008 Feb 20, http://dx.doi.org/10.1016/j.ophtha.2007.10.031.
- Lee P.P., Walt J.W., Rosenblatt L.C., et al. Association between intraocular pressure variation and glaucoma progression: data from a United States chart review. Am J Ophthalmol 2007 Dec; 144(6): 901–907. Epub 2007 Oct 24.
- Smetankin I.G. Dinamika vnutriglaznogo davleniya u patsientov s pervichnoy otkrytougol’noy glaukomoy posle fakoemul’sifikatsii katarakty s ispol’zovaniem koaksial’noy i bimanual’noy metodik [Postoperative intraocular pressure after phaco surgery: bimanual microincision sleeveless phacoemulsification — coaxial phacoemulsification]. Glaukoma — Glaucoma 2008; 4: 32–35.
- Burr J., Azuara-Blanco A., Avenell A., Tuulonen A. Medical versus surgical interventions for open angle glaucoma. Cochrane Database Syst Rev 2012 Sep 12; 9: CD004399, http://dx.doi.org/10.1002/14651858.CD004399.pub3.
- Sharaawy T., Bhartiya S. Surgical management of glaucoma: evolving paradigms. Indian J Ophthalmol 2011; 59: 123–130, http://dx.doi.org/10.4103/0301-4738.73692.
- Razeghinejad M.R., Spaeth G.L. A history of the surgical management of glaucoma. Optom Vis Sci 2011 Jan; 88(1): E39–47, http://dx.doi.org/10.1097/OPX.0b013e3181fe2226.
- Fedorov S.N., Ioffe D.I., Ronkina T.I. Antiglaukomatoznaya operatsiya — glubokaya sklerektomiya [Anti-glaucomatous surgery — deep sclerectomy]. Vestnik oftal’mologii — Vestnik of Ophthalmology 1982; 4: 6–10.
- Fedorov S.N., Kozlov V.I., Timoshkina N.T. Nepronikayushchaya glubokaya sklerektomiya pri otkrytougol’noy glaukome [Non-penetrating deep sclerectomy in open-angle glaucoma]. Oftal’mokhirurgiya — Ophthalmosurgery 1989; 3/4: 52–55.
- Krasnov M.M. Mikrokhirurgiya glaukom [Glaucoma microsurgery]. Moscow: Meditsina; 1980; 248 p.
- Neroev V.V., Bykov V.P., Kvasha O.I., Belevtseva T.A. Khirurgicheskoe lechenie glaukomy putem mikrodrenirovaniya [Micro draining surgery in glaucoma treatment]. Russkiy meditsinskiy zhurnal Prilozhenie. Klinicheskaya oftal’mologiya — Russian medical journal. Supplement. Clinical ophthalmology 2009; 10(3): 113–116.
- Patel S., Pasquale L.R. Glaucoma drainage devices: a review of the past, present, and future. Semin Ophthalmol 2010 Sep–Nov; 25(5–6): 265–270, http://dx.doi.org/10.3109/08820538.2010.518840.
- Gedde S.J., Parrish R.K. 2nd, Budenz D.L., Heuer D.K. Update on aqueous shunts. Exp Eye Res 2011 Sep; 93(3): 284–290. Epub 2011 Mar 31, http://dx.doi.org/10.1016/j.exer.2011.03.013.
- Minckler D.S., Francis B.A., Hodapp E.A., et al. Aqueous shunts in glaucoma: a report by the American Academy of Ophthalmology. Ophthalmology 2008 Jun; 115(6): 1089–1098, http://dx.doi.org/10.1016/j.ophtha.2008.03.031.
- Kahook M.Y. Glaucoma surgery: how do we get from here to there? Middle East Afr J Ophthalmol 2009 Jul–Sep; 16(3): 105–106, http://dx.doi.org/10.4103/0974-9233.56218.
- Souza C., Tran D.H., Loman J., et al. Long-term outcomes of Ahmed glaucoma valve implantation in refractory glaucomas. Am J Ophthalmol 2007 Dec; 144(6): 893–900. Epub 2007 Oct 4.
- Gedde S.J., Panarelli J.F., Banitt M.R., Lee R.K. Evidenced-based comparison of aqueous shunts. Curr Opin Ophthalmol 2013 Mar; 24(2): 87–95, http://dx.doi.org/10.1097/ICU.0b013e32835cf0f5.
- Yu D.Y., Morgan W.H., Sun X., et al. The critical role of the conjunctiva in glaucoma filtration surgery. Prog Retin Eye Res 2009 Sep; 28(5): 303–328. Epub 2009 Jun 30, http://dx.doi.org/10.1016/j.preteyeres.2009.06.004.
- Schmidt W., Kastner C., Sternberg K., et al. New concepts for glaucoma implants — controlled aqueous humor drainage, encapsulation prevention and local drug delivery. Curr Pharm Biotechnol 2013 Jan; 14(1): 98–111.
- Nguyen Q.H., Budenz D.L., Parrish R.K. 2nd. Complications of Baerveldt glaucoma drainage implants. Arch Ophthalmol 1998 May; 116(5): 571–575, http://dx.doi.org/10.1001/archopht.116.5.571.
- Minckler D.S., Hill R.A. Use of novel devices for control of intraocular pressure. Exp Eye Res 2009 Apr; 88(4): 792–798. Epub 2008 Nov 30, http://dx.doi.org/10.1016/j.exer.2008.11.010.
- Saheb H., Ahmed I.I. Micro-invasive glaucoma surgery: current perspectives and future directions. Curr Opin Ophthalmol 2012 Mar; 23(2): 96–104, http://dx.doi.org/10.1097/ICU.0b013e32834ff1e7.
- Erichev V.P., Asratyan G.K. Effektivnost’ i bezopasnost’ mikroshuntirovaniya v khirurgii pervichnoy glaukomy [Efficiency and safety in primary glaucoma surgery]. Glaukoma — Glaucoma 2012; 4: 50–54.
- Rouse J.M., Sarkisian S.R. Jr. Mini-drainage devices: the Ex-PRESS Mini-Glaucoma Device. Dev Ophthalmol 2012; 50: 90–95. Epub 2012 Apr 17, http://dx.doi.org/10.1159/000334780.
- Gregory L., Khouri A.S., Lari H.B., Fechtner R.D. Technique for intraoperative reuse of Ex-PRESS delivery system. J Glaucoma 2013 Apr–May; 22(4): e5–e6, http://dx.doi.org/10.1097/IJG.0b013e318239c1bd.
- Khouri A.S., Khan M.N., Fechtner R.D., Vold S.D. Technique for removal of malpositioned Ex-PRESS glaucoma device. J Glaucoma 2012 Dec 3. [Epub ahead of print].
- Hoffman R.S., Crandall A.S., Crandall D.A., et al. Minimally invasive external mini-glaucoma shunt implantation without conjunctival dissection. J Glaucoma 2012 Aug 23. [Epub ahead of print], http://dx.doi.org/10.1097/IJG.0b013e31826a7edf.
- Salim S. Current variations of glaucoma filtration surgery. Curr Opin Ophthalmol 2012 Mar; 23(2): 89–95, http:// dx.doi.org/10.1097/ICU.0b013e32834ff401.
- Seider M.I., Rofagha S., Lin S.C., Stamper R.L. Resident-performed Ex-PRESS shunt implantation versus trabeculectomy. Journal of Glaucoma 2012 Sep; 21(7): 469–474, http://dx.doi.org/10.1097/IJG.0b013e3182182bfb.
- Dahan E., Ben Simon G.J., Lafuma A. Comparison of trabeculectomy and Ex-PRESS implantation in fellow eyes of the same patient: a prospective, randomised study. Eye (Lond) 2012 May; 26(5): 703–710. Published online 2012 Feb 17, http://dx.doi.org/10.1038/eye.2012.13.
- Hammel N., Lusky M., Kaiserman I., Robinson A., Bahar I. Changes in anterior segment parameters after insertion of Ex-PRESS miniature glaucoma implant. J Glaucoma 2012 Mar 7. [Epub ahead of print], http://dx.doi.org/10.1097/IJG.0b013e31824d4fa1.
- Ashley E. Laing, Leonard K. Seibold, Jeffrey R. SooHoo, Malik Y. Kahook. Evaluation of bleb characteristics after implantation of the EX-PRESS™ glaucoma filtration device. Mol Vis 2012; 18: 10–13. Published online 2012 Jan 4.
- Aziz H., Fantes F., Dubovy S. Histopathology of the Ex-PRESS shunt. Ophthalmic Surgery, Lasers and Imaging Retina 2011; 42: e94–e6, http://dx.doi.org/10.3928/15428877-20110922-01.
- De Feo F., Jacobson S., Nyska A., Pagani P., Traverso C.E. Histological biocompatibility of a stainless steel miniature glaucoma drainage device in humans: a case report. Toxicol Pathol 2009 Jun; 37(4): 512–516, http://dx.doi.org/10.1177/0192623309336150.
- Geffen N., Trope G.E., Alasbali T., et al. Is the Ex-PRESS glaucoma shunt magnetic resonance imaging safe? J Glaucoma 2010 Feb; 19(2): 116–168.
- Seibold L.K., Rorrer R.A., Kahook M.Y. MRI of the Ex-PRESS stainless steel glaucoma drainage device. Br J Ophthalmol 2011 Feb; 95(2): 251–254. Epub 2010 Jun 24, http://dx.doi.org/10.1136/bjo.2009.173906.
- Francis B.A., Winarko J. Ab interno Schlemm’s canal surgery: trabectome and I-stent. Dev Ophthalmol 2012; 50: 125–136. Epub 2012 Apr 17, http://dx.doi.org/10.1159/000334794.
- Filippopoulos T., Rhee D.J. Novel surgical procedures in glaucoma: advances in penetrating glaucoma surgery. Curr Opin Ophthalmol 2008 Mar; 19(2): 149–154, http://dx.doi.org/10.1097/ICU.0b013e3282f4f49e.
- Nichamin L.D. Glaukos iStent trabecular micro-bypass. Middle East Afr J Ophthalmol 2009 Jul–Sep; 16(3): 138–140, http://dx.doi.org/10.4103/0974-9233.56227.
- Grieshaber M.C. Ab externo Schlemm’s canal surgery: viscocanalostomy and canaloplasty. Dev Ophthalmol 2012; 50: 109–124. Epub 2012 Apr 17, http://dx.doi.org/10.1159/000334793.
- Smetankin I.G. Opyt primeneniya mikrorazrezov pri odnomomentnom kombinirovannom khirurgicheskom lechenii katarakty, oslozhnennoy otkrytougol’noy glaukomoy [Microincisions using in case of combined procedure: glaucoma and cataract treatment]. Glaukoma — Glaucoma 2009; 2: 72–73.
- Khaimi M.A. Canaloplasty using iTrack 250 microcatheter with suture tensioning on schlemm’s canal. Middle East Afr J Ophthalmol 2009 Jul–Sep; 16(3): 127–129, http://dx.doi.org/10.4103/0974-9233.56224.
- Shingeleton B., Tetz M., Korber N. Circumferential viscodilation and tensioning of Schlemm canal (canaloplasty) with temporal clear corneal phacoemulsification cataract surgery for open-angle glaucoma and visually significant cataract. J Cataract Refract Surg 2008; 34: 433–40, http://dx.doi.org/10.1016/j.jcrs.2007.11.029.
- Godfrey D.G., Fellman R.L., Neelakantan A. Canal surgery in adult glaucomas. Curr Opin Ophthalmol 2009 Mar; 20(2): 116–121, http://dx.doi.org/10.1097/ICU.0b013e32831eef65.
- Mosaed S., Dustin L., Minckler D.S. Comparative outcomes between newer and older surgeries for glaucoma. Trans Am Ophthalmol Soc 2009 Dec; 107: 127–133.
- Cheng J-W., Cheng S.W., Cai J.P., et al. Systematic overview of the efficacy of nonpenetrating glaucoma surgery in the treatment of open angle glaucoma. Med Sci Monit 2011; 17(7): 155–163, http://dx.doi.org/10.12659/MSM.881840.
- Melamed S., Ben Simon G.J., Goldenfeld M., Simon G. Efficacy and safety of gold micro shunt implantation to the supraciliary space in patients with glaucoma: a pilot study. Arch Ophthalmol 2009 Mar; 127(3): 264–269, http://dx.doi.org/10.1001/archophthalmol.2008.611.
- Hoeh H., Ahmed I.I., Grisanti S., et al. Early postoperative safety and surgical outcomes after implantation of a suprachoroidal micro-stent for the treatment of open-angle glaucoma concomitant with cataract surgery. J Cataract Refract Surg 2013 Mar; 39(3): 431–437. http://dx.doi.org/10.1016/j.jcrs.2012.10.040.
- Gulati V., Fan S., Hays C.L., et al. A novel 8-mm Schlemm’s canal scaffold reduces outflow resistance in a human anterior segment perfusion model. Invest Ophthalmol Vis Sci 2013 Mar 5; 54(3): 1698–704, http://dx.doi.org/10.1167/iovs.12-11373.
- Boland M.V., Ervin A.M., Friedman D., Jampel H., Hawkins B., Volenweider D., Chelladurai Y., Ward D., Suarez-Cuervo C., Robinson K.A. Treatment for glaucoma: comparative effectiveness. Comparative effectiveness review No. 60 (Prepared by the Johns Hopkins University Evidence-based Practice Center under Contract No. HHSA 290-2007-10061-I). AHRQ Publication No. 12-EHC038-EF. Rockville (MD): Agency for Healthcare Research and Quality; 2012, http://www.effectivehealthcare.ahrq.gov/reports/final.cfm.
- Boland M.V., Ervin A.M., Friedman D.S., Jampel H.D., Hawkins B.S., Vollenweider D., Chelladurai Y., Ward D., Suarez-Cuervo C., Robinson K.A. Comparative effectiveness of treatments for open-angle glaucoma: a systematic review for the U.S. Preventive Services Task Force. Ann Intern Med 2013 Feb 19; 158(4): 271–279, http://dx.doi.org/10.7326/0003-4819-158-4-201302190-00008.
- Francis B.A., Singh K., Lin S.C., et al. Novel glaucoma procedures: a report by the American Academy of Ophthalmology. Ophthalmology 2011 Jul; 118(7): 1466–1480.
- Reinthal E.K., Rohrbach J.M., Grisanti S. Glaucoma drainage implants. Klin Monbl Augenheilkd 2010 Jan; 227(1): 49–55. Epub 2010 Jan 20, http://dx.doi.org/10.1055/s-0028-1109789.










