Cerebral Infrared Oximetry in Intracranial Hemorrhage
There have been presented up-to-date data on cerebral infrared oximetry application in intracranial hemorrhage. The principles of the technique enabling to perform noninvasive monitoring of cerebral tissue oxygenation have been given. There has been shown the comparability of cerebral oximetry and invasive assessment techniques of cerebral tissue saturation, jugular oximetry and cerebral microcirculation. Some systems for oxygen status determination have been presented. Special attention has been paid to the use of coefficients and indices of cerebral infrared oximetry to assess functional state of cerebral microvasculature and cerebral autoregulation.
There have been described prospects for further development of cerebral oximetry as a part of many-component monitoring in craniocerebral injury and hemorrhagic strokes.
Infrared oximetry (synonym — infrared spectrometry) is associated with F. Jobsis, who was the first to apply it in vivo in 1977. He showed radiation intensity changes to correlate with the concentration of natural chromophores: oxyhemoglobin, deoxyhemoglobin, cytochrome oxydase, melanin, etc [1, 2].
At first, infrared oximetry was not quantitative and showed the tendency for oxygenation change only, and recorded signals had significant fluctuations and tendency to artifacts [3, 4].
Further development of the technique led to tissue oximetry and cerebral infrared oximetry (CO) in particular, as a result of which a routine assessment of oxygen status of cerebral parenchyma [5–9] in various cerebral pathologies became possible. It enabled to use CO as one of neuromonitoring methods.
CO technique is based on the effect of light (wavelength from 680 to 1000 nm) penetration through human tissues, and light absorption by natural chromophores: oxyhemoglobin (HbO2), deoxyhemoglobin (HHb) and cytochrome oxydase. Infrared radiation is delivered from the source via fiber-optic cable (the so called optode) to skin sensors (detectors) consisting of an emitter and a transmitter. These sensors are located symmetrically in relation to midline and spaced 3.5–6 cm apart from one another (Fig. 1) [10].
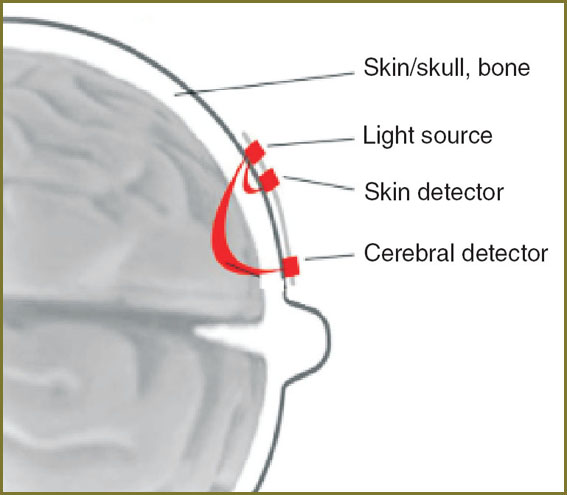 Fig. 1. Cerebral oximetry use scheme Fig. 1. Cerebral oximetry use scheme
|
A light beam from a transmitter penetrates through soft coverings of the head, cranial bones, cerebral parenchyma, and scattering falls on an emitter.
Concentration of chromophores: HbO2, HHb and cytochrome oxydase — is found to be a variable value and depend on the level of tissue oxygenation and metabolism [11]. Concentration of other light-absorbing substances, such as melanin and bilirubin, and other water-soluble fractions, has a trace character, and can be out of calculations [12, 13].
Bouguer–Beer–Lambert modified formula I=I0e–kλl is used to calculate the concentration of chromophores [11,14–16].
A monochromic light beam with intensity I0 passing through an absorbing substance layer, with thicknessI comes out attenuated up to intensity I determined by the following expression I=I0e–kλl, where kλ — index of absorption — coefficient dependent on wavelength λ of absorbed light.
This calculation method is completely applicable in neonatology, since children skull is rather thin to be examined by light from one side to another [15, 16]. However, in adults relative thickness of scalp, cranial bones and brain makes a standard spectroscopy impossible, therefore, CO should be used in a reflection mode, when an emitter and a transmitter are situated on one head side. CO in reflection mode depends on a part of the light, which passes through brain tissue. Human head consists of several layers of different tissues, which exhibit different scattering properties and have different concentrations of compounds absorbing light. As a result, for correct chromophore determination in brain tissues, the introduction of nonlinear coefficients to determine light absorption and scattering was required [16]. Moreover, in order to exclude from calculations the blood in cranial integument, the use of dual receivers situated 2.5–3 cm apart from one another has been suggested recently (See Fig. 1).
At present, several unique CO indices are offered:
1. rSO2 (regional saturation O2) — INVOS and EQUANOX monitors manufactured by Covidien and Nonin Medical (USA), respectively;
2. TOI (Tissue Oxygenation Index) — NIRO monitor manufactured by Hamamatsu Photonics (Japan);
3. rSctO2 (regional cerebral tissue saturation O2) — Fore-Sight monitor manufactured by Casmed (USA).
A lot of studies have shown high degree of reliability of the indices represented that makes oxygen status monitoring a standard procedure [17].
For additional assessment of cerebral oxygen status there have been offered various coefficients and indices showing functional state of microcirculatory bloodstream and cerebral autoregulation:
1) hemispheric asymmetry coefficient — ratio of saturation difference of both hemispheres to their smaller value, expressed as a percentage [8, 18];
2) hemodynamic compliance index — CO values-to-mean AP ratio [19];
3) cerebrovascular reactivity of cerebral saturation index (ТОx);
4) total hemoglobin reactivity index (THx), etc.
CO has found its general use in the assessment of changes in brain regional oxygenation and oxygen status in traumatic brain injury [20–22] and cerebrovascular pathology [3, 6, 23], as well as in patients with pathologies of carotid arteries [24].
Cerebral saturation changes in patients with intracranial hemorrhages are found to correlate significantly with oxygenation changes in the bulb of jugular vein (SjvO2), as well as with brain tissue oxygen tension according to invasive cerebral tissue oximetry (PbtO2) [25, 26]. Cerebral autoregulation in patients with traumatic brain injury and hemorrhagic strokes based on the comparison of reactivity indices has shown that total hemoglobin reactivity index (THx) has highly reliable relationship with intracranial pressure reactivity index (PRx) [19]. In addition, there has been found a direct significant correlation between other cerebral autoregulation indices: cerebral saturation reactivity index (ТОx) and linear cerebral blood flow reactivity index (Sxa) indicating a high accuracy and safety of CO findings [27].
The results obtained by THx and ТОх in about half of patients enabled to determine “optimal” cerebral perfusion pressure (CPPopt) [19, 28] that made CO indispensible in targeted therapy improvement in patients with intracranial hemorrhages, particularly in cases when intracranial pressure monitoring is impossible for some reason [29–32]. CO application enables to estimate brain perfusion changes non-invasively [33–37].
P. Taussky et al. [38] studied the correlation between the parameters of computed tomography brain perfusion and cerebral oxygenation level in patients with non-traumatic intracranial hemorrhages and revealed a highly reliable correlation between SсtO2 and volumetric CBF. Similar data were obtained when comparing CO and positron emission tomography findings [39].
It should be noted that by now no ideal technique has been found to measure the regional total cerebral blood volume (CBV) or cerebral blood flow (CBF) bedside and effectively, quickly, repeatedly and non-invasively. The existing methods applied to measure CBF are technically difficult, laborious, and use radioactive materials or require transportation of patients to perform brain imaging [40].
On the other hand, since CO can measure HHb and HbO2, it is possible to measure total hemoglobin (THgb) [33, 35, 36, 41]. On the assumption that the concentration of hemoglobins is the combination of a larger number of smaller levels of hematocrit, and this correlation is constant during the investigation, and changes in THgb mean CBV change according to the following equation: ΔCBV=[THgb]·(0.89/Hgb).
Thus, it became possible to make a noninvasive assessment of brain perfusion, and it was used in the management of patients with non-traumatic subarachnoid hemorrhages and showed high reliability [39, 42–45].
At the same time, the findings of cerebral perfusion computed tomography and CO in patients with traumatic brain injury, on the contrary, showed a reliable correlation between cerebral oxygenation level and regional CBV [46]. The authors explained the peculiarities of brain perfusion and cerebral oxygenation in traumatic and vascular injury of brain by the fact that regional CBF in contrast to regional blood volume can also depend on arterial bed condition, and therefore, can vary significantly in cerebral angiospasm.
Moreover, CO introduction in clinical practice enabled to find a number of its use limitations. Soft tissue hemorrhages and skull fractures were shown to result in local change of natural chromophore concentration preventing from correct determination of regional saturation in brain parenchyma [47, 48]. Similar errors are described if sensors are located in the areas of high concentration of hair follicles, in sinuses and frontal sinuses [8].
As far as CO measures saturation of brain pooled blood (in arteries, veins and capillaries), it is still impossible to determine separate saturation of gray and white matter [8, 19], as well as to perform CO and magnetic resonance imaging simultaneously [15, 16]. Moreover, the existing cerebral oximeters are incompatible with МРТ that limits their application in simultaneous investigations [15, 16].
Some researchers [22] suppose that critical intracranial hypertension can reduce the accuracy of brain saturation measurements related to impaired venous outflow and development of vasogenic brain edema.
Finally, CO limitations are individual changes in chromophore levels that reduce the accuracy of cerebral saturation absolute values, therefore, until recently, only the dynamics of indices was of practical importance. However, developed in recent years cerebral infrared spectroscopy using coherent-light sources (lasers) has improved significantly monitoring results and enabled some researchers to position such devices as “an absolute cerebral oximeter” [26].
It is worth mentioning that cerebral oximetry is a fast-developing technology, which has the potential for technological elaboration. The technique improvement, accuracy and specificity increase will expand its application in clinical practice. CO is not only a promising, cheap, non-invasive bedside technique used for volumetric CBF measuring, but also the basis for brain function and structure mapping [40, 49–52].
There are the prospects of development and introduction into practice of so called hybrid devices combining electroencephalography and CO. In English literature such devices are called “Brain-Computer Interface”. They make it possible to increase resolution of brain functional state mapping (Fig. 2) [53–59].
 Fig. 2. Appearance of a prospective hybrid device — Brain-Computer Interface [55] Fig. 2. Appearance of a prospective hybrid device — Brain-Computer Interface [55]
|
The same objective is pursued by CO integration into control and imaging systems, such as computed tomography, magnetic resonance imaging, and duplex ultrasonic units [4]. Portable CO devices integrated with wireless telemetry are being tested [42].
High hopes are put on such technologies as cerebral infrared spectroscopy with temporal, phase and spatial resolution [60–63].
Conclusion. Cerebral infrared spectroscopy has a number of advantages over other monitoring techniques. It provides continuous non-invasive control of brain oxygen status, being relatively easy to use and at the same time – rather sensitive to record brain oxygenation changes.
Real time monitoring of brain tissue saturation changes using infrared spectroscopy enables to detect early critical ischemic events, before they are manifested clinically. It makes the technique feasible in modern complex of neuromonitoring.
Study Funding. The work was supported by Territorial Compulsory Medical Insurance Fund.
Conflict of Interests. The authors have no conflicts of interest to declare.
References
- Jobsis F.F. Non-invasive infrared monitoring of cerebral and myocardial oxygen sufficiency and circulatory parameters. Science 1977(4323); 198: 1264–1267, http://dx.doi.org/10.1126/science.929199.
- Gersten A., Perle J., Raz A., Fried R. Probing brain oxygenation with near infrared spectroscopy. NeuroQuantology 2009; 7(2): 258–266, http://dx.doi.org/10.14704/nq.2009.7.2.229.
- Dylst D., De Deyne C.S., Weyns F., Jans F., Heylen R. Monitoring of absolute cerebral oxygen saturation during craniotomy for acute intracerebral bleeding. Eur J Anaesthesiol 2009; 26(Suppl 45): 5–6.
- Cutini S., Moro S.B., Bisconti S. Functional near infrared optical imaging in cognitive neuroscience: an introductory review. J Near Infrared Spectrosc 2012; 20: 75–92, http://dx.doi.org/10.1255/jnirs.969.
- Highton D., Elwell C., Smith M. Noninvasive cerebral oximetry: is there light at the end of the tunnel? Curr Opin Anaesthesiol 2010; 23(5): 576–581.
- Matskeplishvili M.T. Tserebral’naya oksimetriya v kompleksnom neinvazivnom monitoringe tserebral’nykh funktsiy u bol’nykh v ostroy stadii polusharnogo insul’ta. Avtoref. dis. ... kand. med. nauk [Cerebral oximetry in a complex noninvasive monitoring of cerebral functions in patients with acute hemispheric stroke. Abstract for Dissertation for the degree of Candidate of Medical Science]. Moscow; 2012.
- Bauernfeind G., Leeb R., Wriessnegger S.C., Pfurtscheller G. Development, set-up and first results for a one-channel near-infrared spectroscopy system. Biomed Tech (Berl) 2008; 53(1): 36–43, http://dx.doi.org/10.1515/BMT.2008.005.
- Lazarev V.V. Tserebral’naya oksimetriya i neyromonitoring v diagnostike vtorichnykh povrezhdeniy golovnogo mozga posle vnutricherepnykh krovoizliyaniy. Avtoref. dis. ... kand. med. nauk [Cerebral oximetry and neuromonitoring in diagnostics of secondary brain injuries after intracranial hemorrhage. Abstract for Dissertation for the degree of Candidate of Medical Science]. Moscow; 2004.
- Abdelnour A.F., Huppert T. Real-time imaging of human brain function by near-infrared spectroscopy using an adaptive general linear model. NeuroImage 2009; 46(1): 133–143, http://dx.doi.org/10.1016/j.neuroimage.2009.01.033.
- Rodriguez A., Lisboa T., Martin-Loeches I., Díaz E., Trefler S., Restrepo M.I., Rello J. Mortality and regional oxygen saturation index in septic shock patients: a pilot study. J Trauma 2011; 70(5): 1145–1152, http://dx.doi.org/10.1097/TA.0b013e318216f72c.
- Komiyama T., Quaresima V., Shigematsu H., Ferrari M. Comparison of two spatially resolved near-infrared photometers in the detection of tissue oxygen saturation: poor reliability at very low oxygen saturation. Clin Sci (Lond) 2001; 101(6): 715–718.
- De Backer D., Ospina-Tascon G., Salgado D., Favory R., Creteur J., Vincent J.L. Monitoring the microcirculation in the critically ill patient: current methods and future approaches. Intensive Care Med 2010; 36(11): 1813–1825, http://dx.doi.org/10.1007/s00134-010-2005-3.
- Durduran T., Choe R., Baker W.B., Yodh A.G. Diffuse optics for tissue monitoring and tomography. Rep Prog Phys 2010: 73: 076701, http://dx.doi.org/10.1088/0034-4885/73/7/076701.
- Toet M.C., Lemmers P.M.A. Brain monitoring in neonates. Early Hum Dev 2009; 85(2): 77–84, http://dx.doi.org/10.1016/j.earlhumdev.2008.11.007.
- Kleinschmidt A., Obrig H., Requardt M., Merboldt K.D., Dirnagl U., Villringer A., Frahm J. Simultaneous recording of cerebral blood oxygenation changes during human brain activation by magnetic resonance imaging and near-infrared spectroscopy. J Cereb Blood Flow Metab 1996; 16(5): 817–826, http://dx.doi.org/10.1097/00004647-199609000-00006.
- Quaresima V., Bisconti S., Ferrari M. A brief review on the use of functional near-infrared spectroscopy (fNIRS) for language imaging studies in human newborns and adults. Brain Lang 2012; 121(2): 79–89, http://dx.doi.org/10.1016/j.bandl.2011.03.009.
- Moerman A., Wouters P. Near-infrared spectroscopy monitoring in contemporary anesthesia and critical care. Acta Anaesthesiol Belg 2010; 61(4): 185–194.
- Drayna P.C., Abramo T.J., Estrada C. Near-infrared spectroscopy in the critical setting. Pediatr Emerg Care 2011; 27(5): 432–439, http://dx.doi.org/10.1097/PEC.0b013e3182188442.
- Zweifel C., Castellani G., Czosnyka M., Helmy A., Manktelow A., Carrera E., et al. Noninvasive monitoring of cerebrovascular reactivity with near infrared spectroscopy in head-injured patients. J Neurotrauma 2010; 27: 1951–1958.
- Tachtsidis I., Tisdall M., Pritchard C., Leung T.S., Ghosh A., Elwell C.E., Smith M. Analysis of the changes in the oxidation of brain tissue cytochrome-c-oxidase in traumatic brain injury patients during hypercapnoea: a broadband NIRS study. Oxygen Transport to Tissue XXXII. Adv Exp Med Biol 2011; 701: 9–14, http://dx.doi.org/10.1007/978-1-4419-7756-4_2.
- Diedler J., Zweifel C., Budohoski K. Assessment of cerebrovascular reactivity using THx depends on power of slow oscillations. In: 14th International Conference Intracranial Pressure and Brain Monitoring; 2010 Sept 12–16. Tbingen, Germany; 2010; p. 145–146.
- Weerakkody R., Czosnyka M., Zweifel C., Castellani G., Smielewski P., Brady K., et al. Near infrared spectroscopy as possible non-invasive monitor of slow vasogenic ICP waves. Intracranial Pressure and Brain Monitoring XIV. Acta Neurochirurgica Supplementum 2012; 114: 181–185, http://dx.doi.org/10.1007/978-3-7091-0956-4_35.
- Mutoh T., Ishikawa T., Suzuki A., et al. Continuous cardiac output and near-infrared spectroscopy monitoring to assist in management of symptomatic cerebral vasospasm after subarachnoid hemorrhage. Neurocrit Care 2010; 13(3): 331–338.
- Heringlake M., Garbers C., Kabler J., Anderson I., Heinze H., Schon J., et al. Preoperative cerebral oxygen saturation and clinical outcomes in cardiac surgery. Anesthesiology 2011; 114: 58–69, http://dx.doi.org/10.1097/ALN.0b013e3181fef34e.
- Budohoski K., Diedler J., Zweifel C. Comparison of changes in brain tissue oxygenation, tissue oxygen index and tissue hemoglobin index in response to transient changes in cerebral hemodynamics In: 14th International Conference Intracranial Pressure and Brain Monitoring; 2010 Sept 12–16. Tbingen, Germany; 2010; p. 148.
- MacLeod D. Simultaneous comparison of FORE-SIGHT and INVOS cerebral oximeters to jugular bulb and arterial CO2-oximetry measurements in healthy volunteers. Anesth Analg 2009; 108(SCA Suppl): 1–104.
- Budohoski K., Czosnyka M., Smielewski P., Varsos G.V., Kasprowicz M., Brady K.M. Cerebral autoregulation after subarachnoid haemorrhage: comparison of three methods. J Cereb Blood Flow Metab 2013 Mar; 33(3): 449–456, http://dx.doi.org/10.1038/jcbfm.2012.189.
- Steiner L., Pfister D., Strebel S., Radolovich D., Smielewski P., Czosnyka M. Near-infrared spectroscopy can monitor dynamic cerebral autoregulation in adults. Neurocrit Care 2009; 10(1): 122–128, http://dx.doi.org/10.1007/s12028-008-9140-5.
- Diedler J., Zweifel C., Budohoski K., Kasprowicz M., Sorrentino E., Haubrich C. The limitations of near-infrared spectroscopy to assess cerebrovascular reactivity: the role of slow frequency oscillations. Anesth Analg 2011 Oct; 113(4): 849–8457, http://dx.doi.org/10.1213/ANE.0b013e3182285dc0.
- Highton D., Panovska-Griffiths J., Ghosh A., Tachtsidis I., Banaji M., Elwell C., Smith M. Modelling cerebrovascular reactivity: a novel near-infrared biomarker of cerebral autoregulation? Oxygen Transport to Tissue XXXIV. Advances in Experimental Medicine and Biology 2013; 765: 87–93, http://dx.doi.org/10.1007/978-1-4614-4989-8_13.
- Kim M., Durduran T., Frangos S., Edlow B.L., Buckley E.M., Moss H.E., et al. Noninvasive measurement of cerebral blood flow and blood oxygenation using near-infrared and diffuse correlation spectroscopies in critically brain-injured adults. Neurocrit Care 2010 Apr; 12(2): 173–180, http://dx.doi.org/10.1007/s12028-009-9305-x.
- Zweifel C., Castellani C. Non-invasive monitoring of cerebrovascular reactivity with near infrared spectroscopy in head injured patients. In: 14th International Conference Intracranial Pressure and Brain Monitoring; 2010 Sept 12–16. Tbingen, Germany; 2010; p. 48–49.
- Gupta C.N., Palaniappan R. Novel analysis techniques for a brain biometric system. Int J Medical Engineering and Informatics 2008; 1(2): 266–273.
- Zweifel C., Castellani G., Czosnyka M. Continuous assessment of cerebral autoregulation with near infrared spectroscopy in adults after subarachnoid hemorrhage. In: 14th International Conference Intracranial Pressure and Brain Monitoring; 2010 Sept 12–16. Tbingen, Germany; 2010; p. 194–195.
- Lemm S., Dickhaus T., Blankertz B., Müller K.R. Introduction to machine learning for brain imaging. NeuroImage 2011; 56(2): 387–399, http://dx.doi.org/10.1016/j.neuroimage.2010.11.004.
- Blankertz B., Dornhege G., Krauledat M., Müller K.R., Curio G. The non-invasive Berlin Brain–Computer Interface: fast acquisition of effective performance in untrained subjects. NeuroImage 2007; 37(2): 539–550, http://dx.doi.org/10.1016/j.neuroimage.2007.01.051.
- Multidetector computed tomography in cerebrovascular disease. Miles K.A., Eastwood J.D., König M. (eds). London: Informa UK; 2007; 192 p.
- Taussky P., O’Neal B., Daugherty W.P., Luke S., Thorpe D., Pooley R.A., et al. Validation of frontal near-infrared spectroscopy as noninvasive bedside monitoring for regional cerebral blood flow in brain-injured patients. Neurosurg Focus 2012; 32(2): e2, http://dx.doi.org/10.3171/2011.12.FOCUS11280.
- Brady K., Joshi B., Zweifel C., Smielewski P., Czosnyka M., Easley R.B., Hogue C.W.Jr. Real-time continuous monitoring of cerebral blood flow autoregulation using near-infrared spectroscopy in patients undergoing cardiopulmonary bypass. Stroke 2010; 41(9): 1951–1956, http://dx.doi.org/10.1161/STROKEAHA.109.575159.
- Orihuela-Espina F., Leff D.R., James D.R.C., Darzi A.W., Yang G.Z. Quality control and assurance in functional near infrared spectroscopy (fNIRS) experimentation. Phys Med Biol 2010; 55(13): 3701–3724, http://dx.doi.org/10.1088/0031-9155/55/13/009.
- Takeuchi M., Hori E., Takamoto K., Tran A.H., Satoru K., Ishikawa A., et al. Brain cortical mapping by simultaneous recording of functional near infrared spectroscopy and electroencephalograms from the whole brain during right median nerve stimulation. Brain Topogr 2009; 22(3): 197–214, http://dx.doi.org/10.1007/s10548-009-0109-2.
- Ou W., Nissilä I., Radhakrishnan H., Boas D.A., Hämäläinen M.S., Franceschini M.A. Study of neurovascular coupling in humans via simultaneous magnetoencephalography and diffuse optical imaging acquisition. NeuroImage 2009; 46(3): 624–632, http://dx.doi.org/10.1016/j.neuroimage.2009.03.008.
- MacLeod D., Ikeda K., White W. Relationship of cerebral oximetry measured hemoglobin per volume of tissue to arterial blood hemoglobin. Anesth Analg 2008; 106: S–120.
- Trofimov A.O., Yur’ev M.Yu., Voennov O.V. Mozgovoy krovotok i tserebral’naya oksigenatsiya u patsientov s cherepno-mozgovoy travmoy. Sopostavlenie dannykh perfuzionnoy komp’yuternoy tomografii i tserebral’noy infrakrasnoy spektroskopii [Cerebral blood flow and cerebral oxygenation in patients with craniocerebral injury. Comparison of perfusion computed tomography and cerebral infrared spectroscopy data]. Ukrainskiy neyrokhirurgicheskiy zhurnal — Ukraine Neurosurgery Journal 2013; 1: 40–45.
- Wolf M., Ferrari M., Quaresima V. Progress of near-infrared spectroscopy and topography for brain and muscle clinical applications. J Biomed Opt 2007; 12(6): 062104, http://dx.doi.org/10.1117/1.2804899.
- Leal-Noval S., Cayuela A., Arellano-Orden V., Marín-Caballos A., Padilla V., Ferrändiz-Millón C., Corcia Y., et al. Invasive and noninvasive assessment of cerebral oxygenation in patients with severe traumatic brain injury. Intensive Care Med 2010; 36: 1309–1317, http://dx.doi.org/10.1007/s00134-010-1920-7.
- Trofimov A.O., Kalentiev G.V., Aleynikov A.V. Ispol’zovanie tserebral’noy oksimetrii v ostrom periode tyazheloy politravmy [The use of cerebral oximetry in acute period of severe polytrauma]. Sovrem Technol Med — Modern Technologies in Medicine 2012; 4: 64–67.
- Smith M. Shedding light on the adult brain: a review of the clinical applications of near-infrared spectroscopy. Phil Trans R Soc A 2011; 36: 4452–4469, http://dx.doi.org/10.1098/rsta.2011.0242.
- Ferrari M., Quaresima V. A brief review on the history of human functional near-infrared spectroscopy (fNIRS) development and fields of application. NeuroImage 2012, http://dx.doi.org/10.1016/j.neuroimage.2012.03.049.
- Herrmann M.J., Huter T., Plichta M.M., Ehlis A.C., Alpers G.W., Mhlberger A., Fallgatter A.J. Enhancement of activity of the primary visual cortex during processing of emotional stimuli as measured with event-related functional near-infrared spectroscopy and event-related potentials. Hum Brain Mapp 2008; 29(1): 28–35, http://dx.doi.org/10.1002/hbm.20368.
- Budohoski K., Zweifel C., Kasprowicz M., Sorrentino E., Diedler J., Brady K.M., et al. What comes first? The dynamics of cerebral oxygenation and blood flow in response to changes in arterial pressure and intracranial pressure after head injury. Br J Anaesth 2012 Jan; 108(1): 89–99, http://dx.doi.org/10.1093/bja/aer324.
- Constantoyannis C., Sakellaropoulos G.C., Kagadis G.C., Katsakiori P.F., Maraziotis T., Nikiforidis G.C., Papadakis N. Transcranial cerebral oximetry and transcranial doppler sonography in patients with ruptured cerebral aneurysms and delayed cerebral vasospasm. Med Sci Monit 2007; 13(10): MT35–40.
- Coyle S., Ward T., Markham C., McDarby G. On the suitability of near-infrared (NIR) systems for next-generation brain-computer interfaces. Physiol Meas 2004; 25(4): 815–822.
- Hirshfield L.M., Chauncey K., Gulotta R., Girouard A., Solovey E.T., Jacob R.J.K., Sassaroli A., Fantini S. Combining electroencephalograph and functional near infrared spectroscopy to explore users’ mental workload. Foundations of Augmented Cognition. Neuroergonomics and Operational Neuroscience. Lecture Notes in Computer Science 2009; 5638: 239–247, http://dx.doi.org/10.1007/978-3-642-02812-0_28.
- Hermes D., Vansteensel M.J., Albers A.M., Bleichner M.G., Benedictus M.R., Mendez Orellana C., Aarnoutse E.J., Ramsey N.F. Functional MRI-based identification of brain areas involved in motor imagery for implantable brain-computer interfaces. J Neural Eng 2011; 8(2): 025007, http://dx.doi.org/10.1088/1741-2560/8/2/025007.
- Kanoh S., Murayama Y.M., Miyamoto K., Yoshinobu T., Kawashima R. A NIRS based brain–computer interface system during motor imagery: system development and online feedback training. Conf Proc IEEE Eng Med Biol Soc 2009; 2009: 594–547, http://dx.doi.org/10.1109/IEMBS.2009.5333710.
- Nagaoka T., Sakatani K., Awano T., Yokose N., Hoshino T., Murata Y., et al. Development of a new rehabilitation system based on a brain-computer interface using near-infrared spectroscopy. Oxygen Transport to Tissue XXXI. Advances in Experimental Medicine and Biology 2010; 662: 497–503, http://dx.doi.org/10.1007/978-1-4419-1241-1_72.
- Wilson J., Palaniappan R. Analogue mouse pointer control via an online steady state visual evoked potential (SSVEP) brain–computer interface. Journal of Neural Engineering 2011; 8(2): 025026, http://dx.doi.org/10.1088/1741-2560/8/2/025026.
- Sitaram R., Zhang H., Guan C., Thulasidas M., Hoshi Y., Ishikawa A., et al. Temporal classification of multichannel near-infrared spectroscopy signals of motor imagery for developing a brain–computer interface. NeuroImage 2007; 34(4): 1416–1427, http://dx.doi.org/10.1016/j.neuroimage.2006.11.005.
- Gratton G., Fabiani M. Fast optical imaging of human brain function. Front Hum Neurosci 2010; 4: 52, http://dx.doi.org/10.3389/fnhum.2010.00052.
- Wriessnegger S.C., Kurzmann J., Neuper C. Spatio-temporal differences in brain oxygenation between movement execution and imagery: a multichannel near-infrared spectroscopy study. Int J Psychophysiol 2008; 67(1): 54–63, http://dx.doi.org/10.1016/j.ijpsycho.2007.10.004.
- Tsubone T., Muroga T., Wada Y. Application to robot control using brain function measurement by near-infrared spectroscopy. Conf Proc IEEE Eng Med Biol Soc 2007; 2007: 5342–5345, http://dx.doi.org/10.1109/IEMBS.2007.4353548.
- Gupta C.N., Palaniappan R., Swaminathan S. Novel analysis technique for a brain biometric system. International Journal of Medical Engineering and Informatics 2008; 1(2): 266–273.










