LED Light Source for in vitro Study of Photosensitizing Agents for Photodynamic Therapy
The aim of the investigation was to develop a LED light source providing a homogeneous light distribution in 96-well plates and allowing an independent irradiation of individual wells, as well as its experimental testing in in vitro study of photosensitizers for photodynamic therapy.
Materials and Methods. The experiments were carried out on human cell lines of epidermoid carcinoma А-431 and human bladder carcinoma Т24. Two photosensitizers for fluorescence diagnostics and photodynamic therapy were used: Photosens®, and newly synthesized polymer brushes nanoparticles doped with porphyrazine chromophore. To study the photo-activity of the agents we created a light source with replaceable LED-arrays. Photodynamic activity of the photosensitizers was estimated in vitro by MTT assay.
Results. The created LED light source enables to expose cell cultures with narrow-band irradiation with different wavelengths and radiation intensity being up to 90 mW/cm2. Precision control of temperature conditions during the investigation is provided. Light power instability is less than 1%. Independent on and off switching of LED clusters of 4 elements is provided in order to illuminate several wells groups of a standard 96-well culture plate with different light doses simultaneously. The developed light source was tested in the study of photo-activity of agents for photodynamic therapy. The dependence of Photosens® toxicity on the light dose was evaluated and significant photodynamic activity of newly synthesized porphyrazine fluorophore was demonstrated.
Conclusion. A newly developed LED light source with replaceable LED-arrays with narrow spectral bands provides an effective in vitro study of photosensitizing preparations under development. The state-of-the-art approach enables high throughput screening of promising agents for fluorescence diagnostics and photodynamic therapy.
The development of drugs for photodynamic therapy requires studying their functional properties on cell cultures. Analysis of photodynamic activity in vitro enables screening potential photosensitizers, selecting proper concentrations, as well as assessing the dose of optical radiation needed for the photodynamic effect to occur.
Despite a great amount of methods for assessing photodynamic activity of potential photosensitizers on cell cultures described in the literature, a unified approach to such studies has not been developed yet. One of the key features by which all known methods of phototoxicity studies can be classified is the choice of a light source. Many research teams use sources of wideband radiation, such as gas-discharge halogen lamps, filament lamps, or “white” light emitting diodes (LED) [1–9]. This approach has a number of drawbacks, the most important of which is an excess light power acting on the cell culture, as the radiation spectrum of white light sources is several times wider than the absorption spectrum of photosensitizers (the typical absorption band is about 50 nm [10–14]). Radiation beyond this spectral band may give rise to undesirable heating of the cells and to side photochemical reactions, which may hinder interpretation of the experimental findings.
To obtain light with required spectral properties, methods of additional spectral filtering may be used. This approach, however, often leads to significant complication of the laboratory system [5, 8, 15].
In clinical practice, narrowband laser and LED light sources are usually used for photodynamic therapy procedures [16, 17]. This is explained, above all, by relatively simple directed transfer of this radiation via flexible optical fibers, providing at the same time the required spectral power density. When working with cell cultures, it is reasonable to use narrowband sources as well, which allow studying photodynamic activity of agents in the conditions maximum close to the clinical ones [18–21].
Another important parameter is the size of the light exposure field, which determines the possibility of working with cultural vessels of different area. Irradiation of cells in Petri dishes with a diameter of 35–60 mm using distributed light sources (light spot more than 1 cm2) [19, 22, 23] greatly hampers experiments with high biological repeatability. This approach requires a larger amount of material used, and is time-consuming due to impossibility of simultaneous exposure of the experimental samples. Therefore, multiwell plates seem to be more convenient [18, 20, 21, 24, 25].
Despite the fact that simultaneous irradiation of the entire plate area provides identical conditions for all wells, it does not allow assessing the impact of different light doses in a single experiment. At the same time, finding the dependence of the value of photodynamic effect on irradiation dose is the necessary step in the study of the properties of potential agents for fluorescence diagnostics and photodynamic therapy.
The goals of the research are creation of a LED light source for obtaining a uniform light flow in 96-well plates permitting independent exposure of individual well groups to light and its experimental testing in in vitro study of light activity of agents for photodynamic therapy.
Materials and Methods
Cell culturing. Cell lines of human epidermoid carcinoma A431 (ATCC® CRL-1555TM, provided by the Institute of Bioorganic Chemistry of the Russian Academy of Sciences, Russia) and human bladder carcinoma T-24 (ATCC® HTB4TM; Research Institute of Virology of the Russian Academy of Medical Sciences, Russia) were used. A-431 cells were cultured in DMEM medium (PanEco, Russia), and T-24 cells in Eagle MEM medium (PanEco, Russia). The growth media contained 10% of fetal bovine serum (HyClone, USA) and 2 mM of L-glutamine (PanEco, Russia). Culturing was performed in a CO2-incubator at 37°C and 5% CO2 atmosphere; the cells were treated with 0.25% solution of trypsin-EDTA (PanEco, Russia) at each passaging stage.
Photosensitizers. The Photosens® drug for fluorescence diagnostics and photodynamic therapy (State Scientific Center “NIOPIC”, Russia) was used. Photosens® is a solution of a mixture of sodium salts of sulfonated aluminum phthalocyanine (from di- to tetrasubstituted) in distilled water [26].
Photoactivity of polymer brushes nanoparticles doped with tetra(4-fluorophenyl)tetracyanoporphyrazine (hereafter referred to as Pz) was investigated [27]. Pz is characterized by the absorption in the red region of the spectrum with a maximum at the wavelength of 613 nm (solution in tetrahydrofuran) and fluorescence with a maximum at 660 nm.
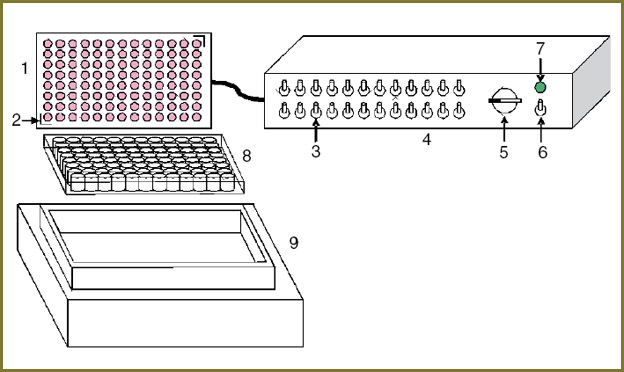
LED light source for a uniform light flow in 96-well plates. For studying the photodynamic activity of PDT agents, a LED light source comprising a plug-in LED array, a control unit and a thermostat was developed (Fig. 1).
96 identical small-size light-emitting diodes for surface mounting (HPL-H44 series, High Power Lighting Corporation, Taiwan) with a 4.4×4.4 mm base and a consumed power of 1 W/chip were used for the LED array. A focusing lens embedded in the LED housing provides effective collection of radiation and formation of a narrow (30°) radiation pattern for miniature array elements. A built-in heat sink permits obtaining high output power (up to 100 mW/chip) with long-term stable operation of the device.
The proposed light source with plug-in LED arrays and narrowband radiation provides effective irradiationof the multiwell plates. In experimental cellular studies, the choice of the central wavelength of LED radiation depends on the spectral absorption characteristics of photosensitizers. The spectral range available for the described methods of LED arrays manufacturing is limited only by the range of powerful small-size surface-mounted LEDs with commercial built-in collimating optics (up to 680 nm in the red spectrum that is most interesting in terms of photodynamic action).
For the experimental approval, two plug-in LED arrays were made meeting the characteristics of spectral absorption of the tested photosensitizers, with central wavelengths of 590 and 625 nm and radiation spectrum width of 20 nm at half-power. Each array was mounted on a massive heat-conducting base providing rigidity of the construction and removal of the heat produced by the LEDs. If high-power radiation is used, an optional radiator with forced air cooling is installed on the array base. The overall array dimensions are the same as for the standard 96-well culturing plate. Each of the miniature LEDs is centrally aligned with the corresponding plate well.
An important distinctive feature of the device is the location of the matrix above the irradiated plate, which allows using the light source with standard thermostats intended for work with multiwell plates and enables precision control of temperature conditions during the experiment. The base of the array is equipped with guiding elements for accurate array installation. The “ThermoStat plus” thermostat (Eppendorf, Germany) has been used in this work.
The control unit is a multiport power supply unit providing smooth power control. The LEDs are grouped into 24 separately commuted linear clusters of 4 elements, each having its own switch on the front panel of the control unit.
The power density of the light flow through the well bottom was measured by the optical power meter PM100A (Thorlabs, USA) with detector head S121C. The detector head during the measurements was placed under the plate well close to its bottom with center-to-center alignment. The registered optical power was normalized to the area of the photosensitive detector region (0.71 cm2). When calculating the averaged values, measurements were made successively for all LEDs in the array.
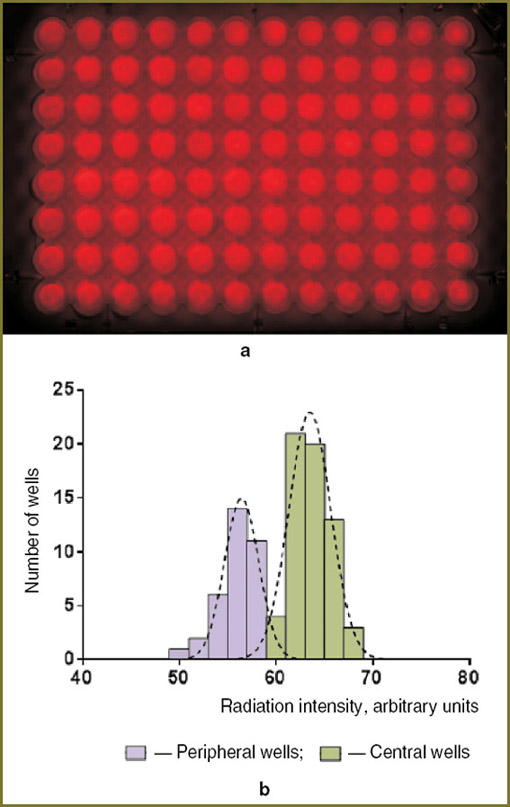
The uniformity of radiation intensity distribution over the area was analyzed using images of the bottom of the plate obtained through a diffuser sheet attached to the bottom in front of the camera. Average intensity for each well was obtained by numerical analysis of the digital photo. The histogram of the radiation intensity distribution averaged over the well area was used to calculate the ratio of the mean values of intensities in the center and at the periphery of the plate.
The density of the power transmitted through the plate well bottom can be set in the range of 1.2–30 mW/cm2 for the 590 nm LEDs, and 2.6–90 mW/cm2 for the 625 nm LEDs. For cell culture studies we used radiation with a spatial power density of 20 mW/cm2 at which thermal effects are not observed yet. Besides, the time of accumulation of the radiation doses used in this work (up to 15 J/cm2) did not exceed 12.5 min, allowing irradiation outside cell incubator.
Analysis of photosensitizer activity. Cells were seeded at a density of 3·103 per well in the 96-well plate and incubated in the CO2-incubator overnight. Then the growth medium in the target wells of the cultural plate was replaced by 200 μl of the medium with photosensitizer and the cells were incubated for 6 or 24 hours for Photosens® or Pz-doped nanoparticles, respectively. After the incubation with the photosensitizer, the medium in the plate well was replaced by the corresponding growth medium. The cells were irradiated by the LED light source. The irradiation dose was controlled by the duration of illumination and amounted to 1.3–15 J/cm2 at the power density of 20 mW/cm2.
The viability of the cell culture was evaluated 24 hours after the irradiation by means of the MTT assay [28]. For this 3(4,5-dimethyl-2-thiasolyl)-2,5-diphenyl-2H-tetrasole bromide (MTT reagent, Alfa Aesar, Great Britain) was added to the growth medium in the final concentration of 0.5 mg/ml and the cells were incubated for 4 hours. Then the incubation medium was removed, and the crystals of the formed stained MTT-formazan were dissolved in 200 μl of dimethylsulfoxide (PanEco, Russia). Optical density of each well content was measured using the microplate reader Synergy MX (BioTek, USA) at the wavelength of 570 nm. The cell viability was assessed by the ratio of the optical density of the formazan solution in each irradiated well and the one of the control well without irradiation. The arithmetic mean and standard deviations are presented on the graphs.
Results
Temporal stability of the characteristics and uniformity of the radiation intensity distribution over plate area. The developed light source with plug-in LED arrays enables irradiation of cell cultures with the spatial intensity up to 90 mW/cm2 at the wavelength of 625 nm and 30 mW/cm2 at 590 nm, providing accumulation of the doses required for the photodynamic effect over relatively short time intervals (units of minutes). The relative intensity variation measured during 30 minutes for the 625 nm matrix at two operation modes (20 and 40 mW/cm2) amounted to 1% (Fig. 2) with the onset time of the operating mode not more than 1 min from the moment of switching on.
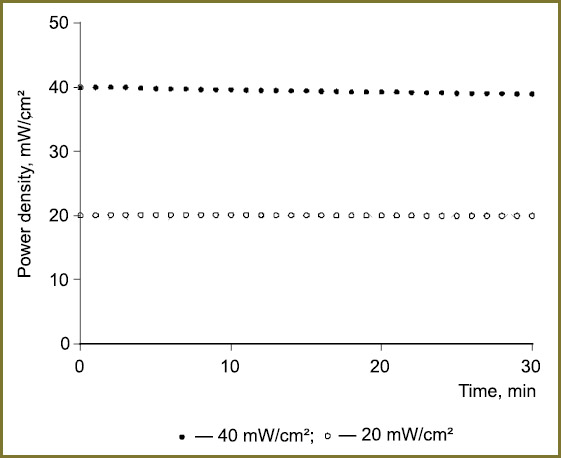 Fig. 2. Temporal stability of radiation of LED light source with 625 nm array after the onset of the operating mode Fig. 2. Temporal stability of radiation of LED light source with 625 nm array after the onset of the operating mode
|
The narrow radiation pattern of the used LED emitters ensures that the main part of the radiation intensity arrives at the bottom of the “target“ well. However, it should be taken into consideration that some radiation is incident on the adjacent wells. Hence, we distinguished the central part of the plate (60 wells) in which all the wells were in identical conditions and rows of the peripheral wells (36 wells). The image of the plate obtained through the diffuser sheet attached to the plate bottom in front of the camera is shown in Fig. 3, a. The numerical analysis of the image (Fig. 3, b) showed that the mean intensity at the periphery of the plate was 10–15% less than in the center. The intensity distribution in the individual plate wells for both zones may be described by the normal distribution law; the relative standard deviation characterizing the spread of values does not exceed 4–5% in both cases.
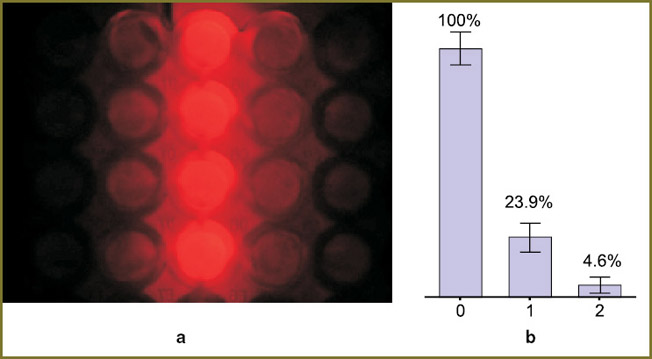
The influence of a single LED cluster radiation on the adjacent plate wells. The light source design provides independent switching of 24 clusters of 4 LEDs each. This allows independent irradiation of the needed well groups. At a constant level of power density in a biological experiment, the irradiation dose may be changed for different well groups by changing the time of exposure to the corresponding LED. Consequently, we studied the impact of the radiation of a single cluster on the adjacent plate wells. It was found that the part of the radiation incident on the adjacent well row is 25% of the radiation in the “target” wells (Fig. 4). The part of the radiation in the next, more remote row does not exceed 4–5%.
The impact of illumination of the adjacent wells, when a single LED cluster was switched on, was verified in the cell experiment: the plate wells were seeded with A-431 cells and preincubated with the Photosens® agent with a concentration of 10–5 mol/L. The plate was illuminated only by one row of LEDs (two clusters arranged in a line), the irradiation dose passing through the bottom of the wells located directly under the LEDs was 15 J/cm2 at the power density of 20 mW/cm2 (Fig. 5).
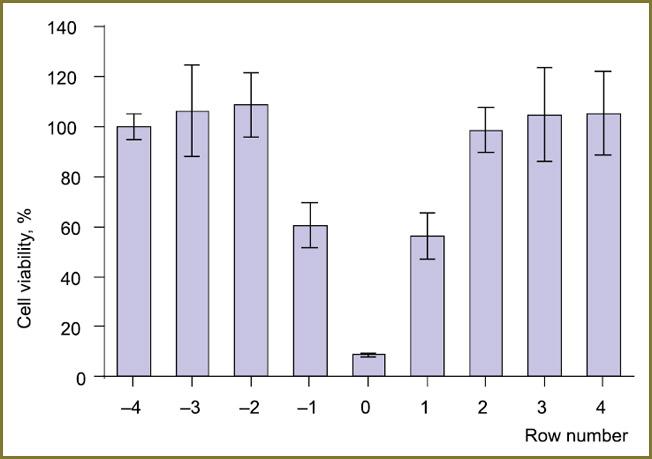 Fig. 5. Viability of A-431 cells preincubated with Photosens® drug as a function of the distance to illuminated well row. MTT assay; radiation dose — 15 J/cm2 Fig. 5. Viability of A-431 cells preincubated with Photosens® drug as a function of the distance to illuminated well row. MTT assay; radiation dose — 15 J/cm2
|
The resulting viability of the cells in the wells under the LEDs was not more than 10% of the control. Significant changes were also observed in the adjacent wells (in Fig. 5 they are designated as “–1” and “1”): at a given concentration of the photosensitizer and the irradiation dose the viability in those wells was only 50–60% of the unexposed variant. At the same time, no biologically significant effect of the individual switched-on LED row on the wells located over 1 row and farther was not observed. Thus, in the case of irradiation of a cell culture in a 96-well plate (12 well rows) it is possible to distinguish several independent well groups and to treat them with different light doses.
To verify identity of simultaneous irradiation of single plate wells and traditional consecutive irradiation of a number of plates, two experiments were conducted using T24 cells preincubated with the Photosens® drug (10–5 mol/L). In the first experiment, the cells cultured on different plates were irradiated by different doses, and in the second one the cells were cultured in the parallel well rows on one and the same plate (Fig. 6). In all the cases the light power density was 20 mW/cm2 and the irradiation dose was varied by the LED operation time. When only one plate was used, different radiation doses were btained by successively switching on the LED clusters: the higher the needed dose, the earlier the corresponding cluster was switched on, whereas all the clusters were switched off together. A good agreement of the data obtained using different approaches to conducting the experiment shows that independent switching of the LED clusters reduces the overall time of the experiment without considerable impact on the results.
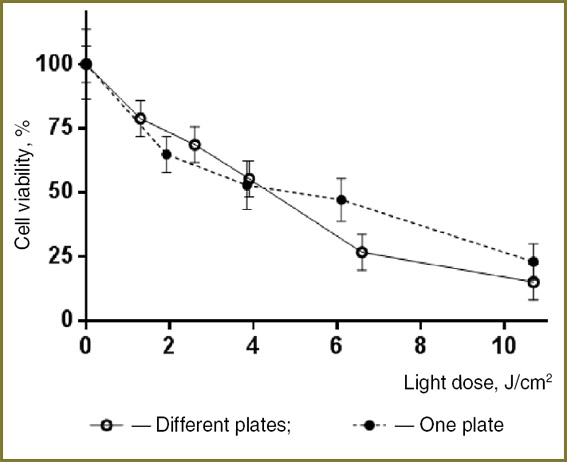 Fig. 6. Light-induced activity of Photosens® drug relative to the cell culture T24 as a function of light dose Fig. 6. Light-induced activity of Photosens® drug relative to the cell culture T24 as a function of light dose
|
Assessment of the photoactivity of polymer nanoparticles doped with porphyrazine chromophore. The developed device was used for studying the photoactivity of nanoparticles based on polymer brushes doped with tetra(4-fluorophenyl) tetracyanoporphyrazine — Pz [27].
A-431 cells were incubated in the medium with the studied nanoparticles (10 and 40 μmol/L Pz) for 24 h, after which they were irradiated using LED arrays at the wavelength of 590 and 625 nm with 10 J/cm2 radiation dose.
The irradiation resulted in dose-dependent reduction of cell viability (Fig. 7); the ratio of the fractions of unviable cells at 625 and 590 nm wavelength was about 1.5, which indicates a higher efficacy of the red (625 nm) radiation in inducing the photodynamic Pz-mediated response.
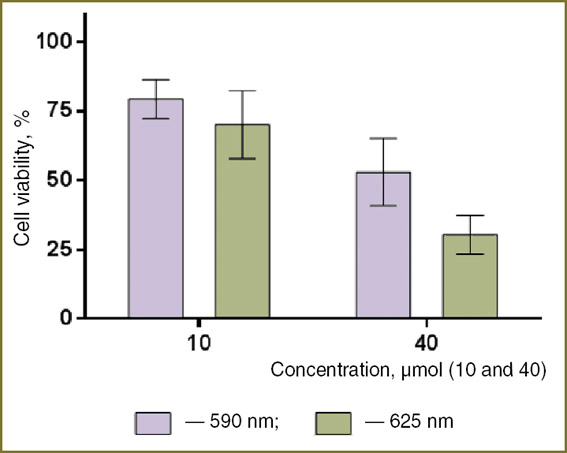 Fig. 7. Viability of A-431 cells preincubated with Pz-doped nanoparticles (10 and 40 μmol/L Pz) and irradiated at the wavelengths of 590 and 625 nm; radiation dose — 10 J/cm2 Fig. 7. Viability of A-431 cells preincubated with Pz-doped nanoparticles (10 and 40 μmol/L Pz) and irradiated at the wavelengths of 590 and 625 nm; radiation dose — 10 J/cm2
|
Discussion. Successful application of the photodynamic therapy along with the revealed drawbacks of the available photosensitizers initiated active search for new agents with high efficacy and minimal side effects. However, despite the high research activity (according to the data of PubMed resource, the number of publications on this topic has been growing year by year) the equipment intended for screening potential agents and investigation of their photoactivity is still very diversified.
The developed light source with interchangeable LED arrays and narrowband radiation described here provides effective irradiation of multiwell plates. The spectrum range is determined by the assortment of the LED models available on the market (up to 680 nm in the red spectrum that is the most interesting in terms of photodynamic action). The use of up-to-date high power small-sized LEDs with built-in optics enabled us to obtain a uniform distribution of radiation intensity both within the area of each well and over the entire central and peripheral parts of the standard 96-well cultural plate. Besides, a narrow radiation pattern and a high power of the used LEDs permitted placing the plug-in LED arrays on the side of the irradiated plate cover and using the device together with standard thermostats for multiwell plates. The proposed geometry ensures precision control of temperature conditions during the studies. This fact together with much higher obtained radiation intensities is a significant advantage of the described device over its close analog [20], in which the LED array is placed directly under the plate bottom. The maximum radiation intensities reported in the work [20] for the sources with central wavelengths of 620 and 625 nm without additional external focusing optics were 10.2 and 44.1 mW/cm2, respectively, which is several times less than the intensities obtained with the LED light source created by the authors of this paper (up to 30 mW/cm2 at 590 nm, and up to 90 mW/cm2 at 625 nm).
The measurements of the influence of a single LED cluster radiation on the adjacent plate wells were confirmed by the experiment on a simulated biological system. The obtained results show that the impact of the switched-on LEDs on the wells located over 1 row from the radiation source is negligible, which allows proposing a scheme of separate switching of the corresponding LED clusters. The trial in the biological experiment using Photosens® showed no biologically significant influence of the switched-on row of the LEDs on the wells located farther than over 1 row.
To reduce the time of testing photoactivity of the potential photosensitizers it is important to assess the effect of different light doses in one and the same experiment (concurrently on one plate). A similar solution was proposed in 2003 in the work [15], where halogen lamp radiation was filtered to a relatively small (620–700 nm) spectral width and focused at the end of the optical fiber bundle which served for directed delivery of light to the target plate wells. However, such a scheme seems to be rather hard to use for a number of reasons. The main thing noted by the authors is, obviously, nonuniform feeding of the optical fibers due to inhomogeneity of the light beam at the end of the fiber bundle. This leads to pronounced spread in the values of the light power incident on separate wells. On the other hand, in this configuration it is impossible to change the irradiated power incident on the individual well and, consequently, the ratio of light doses between separate wells is a strictly fixed value.
The independent commutation of the individual LED clusters permits using a significantly more flexible experimental procedure, when the radiation dose ratio for the well groups is specified randomly, depending on the research task. A good correlation of the results was achieved when the cells were illuminated concurrently on one plate and successively on different plates. This approach enables high throughput screening of new compounds aimed at revealing the most promising ones for fluorescence diagnostics and photodynamic therapy.
Conclusion. The created LED light source produces a uniform light flow in 96-well plates with a precision control of temperature conditions. Irradiation is performed by plug-in LED arrays with different spectral characteristics. Independent switching of the LED clusters of 4 elements enables concurrent irradiation of several well groups of a standard 96-well culture plate with different light doses. The light source was tested in in vitro study of the light activity of agents for photodynamic therapy. The dependence of Photosens® toxicity on the light dose was evaluated and significant photodynamic activity of newly synthesized porphyrazine fluorophore was demonstrated.
Study Funding. The work was partly supported by the Russian Foundation for Basic Research (projects No. 12-04-31730, 12-04-31322, 12-04-33130, 13-04-40228-H, 13-04-92612, 14-0331130, 11-02-00916) and the Ministry of Education and Science (order No. 14.Z50.31.0022). The paper is also based in part on the work financed by the Skolkovo Institute of Science and Technologies (Skoltech) in the frame of the SkolTech/MIT Initiative (project No.203-MRA).
Conflict of Interests. The authors have no conflict of interests to disclose.
Acknowledgments. The authors are grateful to N.M. Shakhova (Institute of Applied Physics of the Russian Academy of Sciences, Nizhny Novgorod) for her valuable ideas and suggestions concerning the design of the device and writing the article, and to L.G. Klapshina (Institute of Molecular Chemistry of the Russian Academy of Sciences, Nizhny Novgorod) for providing polymer nanoparticles doped with porphyrazine chromophore for our study.
References
- Mroz P., Bhaumik J., Dogutan D.K., Aly Z., Kamal Z., Khalid L., Kee H.L., Bocian D.F., Holten D., Lindsey J.S., Hamblin M.R. Imidazole metalloporphyrins as photosensitizers for photodynamic therapy: role of molecular charge, central metal and hydroxyl radical production. Cancer Lett 2009; 282(1): 63–76, http://dx.doi.org/10.1016/j.canlet.2009.02.054.
- Furre I.E., Shahzidi S., Luksiene Z., Moller M.T., Borgen E., Morgan J., Tkacz-Stachowska K., Nesland J.M., Peng Q. Targeting PBR by hexaminolevulinate-mediated photodynamic therapy induces apoptosis through translocation of apoptosis-inducing factor in human leukemia cells. Cancer Res 2005; 65(23): 11051–11060, http://dx.doi.org/10.1158/0008-5472.CAN-05-0510.
- Akhlynina T.V., Jans D.A., Rosenkranz A.A., Statsyuk N.V., Balashova I.Y., Toth G., Pavo I., Rubin A.B., Sobolev A.S. Nuclear targeting of chlorin e6 enhances its photosensitizing activity. J Biol Chem 1997; 272(33): 20328–20331.
- Krieg R.C., Messmann H., Schlottmann K., Endlicher E., Seeger S., Scholmerich J., Knuechel R. Intracellular localization is a cofactor for the phototoxicity of protoporphyrin IX in the gastrointestinal tract: in vitro study. Photochem Photobiol 2003; 78(4): 393–399, http://dx.doi.org/10.1562/0031-8655(2003)0780393ILIACF2.0.CO2
- Feofanov A., Grichine A., Karmakova T., Kazachkina N., Pecherskih E., Yakubovskaya R., Luќyanets E., Derkacheva V., Egret-Charlier M., Vigny P. Chelation with metal is not essential for antitumor photodynamic activity of sulfonated phthalocyanines. Photochem Photobiol 2002; 75(5): 527–533, http://dx.doi.org/10.1562/0031-8655(2002)0750527CWMINE2.0.CO2.
- Tong Z., Singh G., Rainbow A.J. Sustained activation of the extracellular signal-regulated kinase pathway protects cells from photofrin-mediated photodynamic therapy. Cancer Res 2002; 62(19): 5528–5535.
- Gijsens A., Derycke A., Missiaen L., De Vos D., Huwyler J., Eberle A., de Witte P. Targeting of the photocytotoxic compound AlPcS4 to HeLa cells by transferrin conjugated peg-liposomes. Int J Cancer 2002; 101(1): 78–85, http://dx.doi.org/10.1002/ijc.10548.
- Zhukova O.S. In vitro models for scrinning of antitumor compounds of different nature. Rossiyskiy bioterapevticheskiy zhurnal 2004; 3(3): 12–18.
- Berg K., Moan J. Lysosomes as photochemical targets. Int J Cancer 1994; 59(6): 814–822.
- Shirmanova M.V., Balalaeva I.V., Lekanova N.Yu., Mysyagin S.A., Brilkina A.A., Klapshina L.G., Zagaynova E.V. Development of a new photosensitizer based on ytterbium porphyrazine complex. Biofizika 2011; 56(6): 1117–1124.
- Wang X., Wang P., Tong W., Liu Q. Comparison of pharmacokinetics, intracellular localizations and sonodynamic efficacy of endogenous and exogenous protoporphyrin IX in sarcoma 180 cells. Ultrasonics 2010; 50(8): 803–810, http://dx.doi.org/10.1016/j.ultras.2010.04.004.
- Chan W.S., Marshall J.F., Svensen R., Bedwell J., Hart I.R. Effect of sulfonation on the cell and tissue distribution of the photosensitizer aluminum phthalocyanine. Cancer Res 1990; 50(15): 4533–4538.
- Juzenas P., Juzeniene A., Rotomskis R., Moan J. Spectroscopic evidence of monomeric aluminium phthalocyanine tetrasulphonate in aqueous solutions. J Photochem Photobiol B 2004; 75(1–2): 107–110, http://dx.doi.org/10.1016/j.jphotobiol.2004.05.011.
- Sakamoto K., Ohno-Okumura E. Syntheses and functional properties of phthalocyanines. Materials 2009; 2(3): 1127–1179, http://dx.doi.org/10.3390/ma2031127.
- Meerovich I.G., Jerdeva V.V., Meerovich G.A., Derkacheva V.M., Savitsky A.P. High-throughput screening system for the study of phototoxicity of photosensitizers in vitro. SPIE Proc 2003; 4952: 203–208, http://dx.doi.org/10.1117/12.480277.
- Stranadko E.F., Armichev A.V., Geynits A.V. Light sources for photodynamic therapy. Lazernaya meditsina 2011; 15(3): 63–69.
- Mang T.S. Lasers and light sources for PDT: past, present and future. Photodiagnosis and Photodynamic Therapy 2004; 1(1): 43–48.
- Stranadko E.F., Yashunskiy D.V., Khatuntseva E.A., Ustyuzhanina N.E., Ryabov M.V., Ibragimov T.M., Nifant’ev N.E., Gosh R. Looking for new photosensitizers with excitation wavelength in a long-wavelength spectrum. Lazernaya meditsina 2009; 13(1): 29–34.
- Theodossiou T., MacRobert A.J. Comparison of the photodynamic effect of exogenous photoprotoporphyrin and protoporphyrin IX on PAM 212 murine keratinocytes. Photochem Photobiol 2002; 76(5): 530–537, http://dx.doi.org/10.1562/0031-8655(2002)0760530COTPEO2.0.CO2.
- Butler M.C., Itotia P.N., Sullivan J.M. A high-throughput biophotonics instrument to screen for novel ocular photosensitizing therapeutic agents. Invest Ophthalmol Vis Sci 2010; 51(5): 2705–2720, http://dx.doi.org/10.1167/iovs.08-2862.
- Zheng G., Chen J., Stefflova K., Jarvi M., Li H., Wilson B.C. Photodynamic molecular beacon as an activatable photosensitizer based on protease-controlled singlet oxygen quenching and activation. Proc Natl Acad Sci USA 2007; 104(21): 8989–8994, http://dx.doi.org/10.1073/pnas.0611142104.
- Bachor R., Shea C.R., Gillies R., Hasan T. Photosensitized destruction of human bladder carcinoma cells treated with chlorin e6-conjugated microspheres. Proc Natl Acad Sci USA 1991; 88(4): 1580–1584.
- Pogue B.W., Ortel B., Chen N., Redmond R.W., Hasan T. A photobiological and photophysical-based study of phototoxicity of two chlorins. Cancer Res 2001; 61(2): 717–724.
- Vrouenraets M.B., Visser G.W.M., Stigter M., Oppelaar H., Snow G.B., van Dongen G.A.M.S. Targeting of aluminum (III) phthalocyanine tetrasulfonate by use of internalizing monoclonal antibodies. Cancer Res 2001; 61(5); 1970–1975.
- Song K., Li J., Li L., Zhang P., Geng F., Dong R., Yang Q., Qu X., Kong B. Intracellular metabolism, subcellular localization and phototoxicity of HMME/HB in ovarian cancer cells. Anticancer Res 2011; 31(10): 3229–3235.
- Kotel’nikov A.I., Rybkin A.Yu., Goryachev N.S., Belik A.Yu., Kornev A.B., Troshin P.A. Photodynamic activity of hybrid nanostructure based on polycationic derivative of fullerene and phthalocyanine dye Photosense. Doklady Akademii nauk 2013; 452(4): 408–412.
- Yakimansky A.V., Meleshko T.K., Ilgach D.M., Bauman M.A., Anan’eva T.D., Klapshina L.G., Lermontova S.A., Balalaeva I.V., Douglas W.E. Novel regular polyimide-graft-(polymethacrylic acid) brushes: synthesis and possible applications as nanocontainers of cyanoporphyrazine agents for photodynamic therapy. Journal of Polymer Science Part A: Polymer Chemistry 2013; 51(20): 4267–4281, http://dx.doi.org/10.1002/pola.26846.
- Freshni R. Kultura zhivotnykh kletok [Living cell culture]. Moscow: Binom. Laboratoriya znaniy; 2010. 691 p.