Use of Optical Coherence Tomography for Evaluation of the Results of the Treatment of Diabetic Macular Edema with Laser Coagulation of the Ocular Fundus Tissues and Intravitreal Injection of Ranibizumab (Lucentis)
One of the most informative methods of noninvasive investigation of the retina is optical coherence tomography (OCT). OCT is successfully applied to the diagnosis of diabetic retinopathy especially if there is diabetic macular edema (DME).
The objective of the investigation is evaluating the results of the treatment of DME with laser coagulation of the ocular fundus tissues and intravitreal injection of ranibizumab (Lucentis), on the basis of imaging data derived from OCT.
Materials and Methods. Examination, treatment and dynamic observations were carried out on 108 patients (214 eyes at the stage of proliferative diabetic retinopathy with clinically relevant DME); they included 68 women and 40 men. The patients were divided into three groups depending on the overall term of DME occurrence: group I — up to 1 month (73 eyes), group II — from 1 to 3 months (69 eyes), group III — 3 months or longer (72 eyes). Within each group, all the patients were divided into three subgroups which underwent either intravitreal injection of ranibizumab, or laser coagulation of the eye fundus tissues, or both treatments combined. The effectiveness of the treatment was evaluated on the basis of OCT and visometry data recorded at both 5 days and 1 month.
Results. According to OCT of retina, the effect of the intravitreal injection of Lucentis alone was considerable but unstable. In the case of DME having been present for less than 1 month, the combined treatment was the most effective. In cases where DME had been present for 1 to 3 months or for more than 3 months, both laser-only and the combined treatment are effective.
Conclusion. We have therefore shown that choice of optimum treatment is dependent on the previous duration of DME and that OCT is a highly informative method for evaluation of DME.
Introduction. Diabetes mellitus (DM) and its complications are a serious medico-social problem, and globally, in 2010 there were 285 million adults with DM (6.4% of the world population). According to forecasts this figure could increase up to 439 million (7.7% of the population) by 2030 [1].
As the life expectancy of patients with DM has increased, later vascular complications including diabetic retinopathy are of great significance. In developed countries diabetic retinopathy is the leading cause of blindness up to the age of 65 [2].
Diabetic macular edema (DME), developing due to decompensation of the capillary network of the macula, is the most common cause of visual impairment and disability in patients with diabetic retinopathy [3].
To evaluate the changes in the macular region of the retina, optical coherence tomography (OCT) can be used. With this method it is possible to study the thickness and topography of the different retinal layers. According to data from a number of authors, OCT is the most accurate and objective method of monitoring DME [4, 5]. OCT is a noninvasive, contactless method of macula visualization which allows it to be used for screening [6, 7].
Before the introduction of inhibitors of vascular endothelium growth factor (anti-VEGF), laser coagulation was the only standard treatment that could provide sight stabilization of patients with DME [8–10]. Currently anti-VEGF, together with laser therapy, is the standard DME treatment [11]. One of the pharmaceuticals which is an inhibitor of VEGF is ranibizumab (Lucentis).
Various papers have been published in journals regarding the application of different schemes of combined treatment of DME, however there are no data about the effectiveness of DME treatment at different times following its onset [12].
The objective of the investigation was to evaluate the results of the treatment of diabetic macular edema with laser coagulation of the ocular fundus tissues and intravitreal injection of ranibizumab (Lucentis), on the basis of OCT data.
Materials and Methods. Examination, treatment and dynamic observations were carried out on 108 patients (214 eyes at the stage of proliferative diabetic retinopathy with the presence of relevant DME) with insulin-dependent DM of types 1 and 2 at the stage of compensation and subcompensation of the retinal metabolic processes (68 women and 40 men). The age of the patients was 27–75 years. The exclusion criteria were as follows: DM at decompensation of metabolic processes, acute impairment of coronary or cerebral circulation in the anamnesis, severe forms of hypertonia, oncological diseases, and epilepsy. Patients with clinically relevant corneal opacity of the lens and a vitreous body were also excluded.
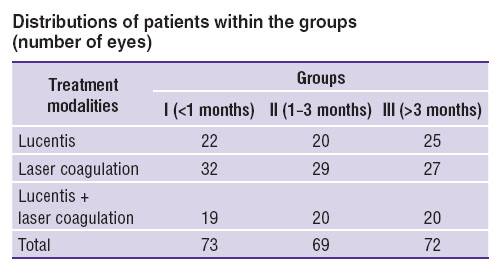
The patients were divided into three groups depending on the term of DME occurrence: group I — up to 1 month from the diagnosis of macular edema (73 eyes), group II — from 1 to 3 months (69 eyes), group III — presence of edema for 3 months and longer (72 eyes). Within each group the patients were divided into subgroups: a, b and с. The patients of subgroup a underwent intravitreal injection of 0.05 ml of Lucentis, b — laser treatment (panretinal laser coagulation of eye fundus tissues), с — combined treatment (injection of Lucentis + laser coagulation of eye fundus tissues) (See the Table).
|
|
The study complies with the declaration of Helsinki and approved by the Ethics Committee of V.I. Razumovsky Saratov State Medical University. Written informed consent was obtained from all patients.
The examination of patients was performed as follows: visometry, Maklakov applanation tonometry, OCT and fluorescent angiography of the retina with Spectralis® OCT (Heidelberg Engineering, Germany). The rate of scanning was 40,000 А-scans per second with a resolution of 3 µm. The rate of scanning together with the use of TruTrackTM technology to track eye movements in real time minimized the occurrence of motion artifacts. The Spectralis® OCT additionally incorporated a system of noise filtration.
Laser coagulation was performed according to the standard method (lattice-type and panretinal) with a Luminous Superscan 577-Y (Quantel Medical, France).
Intravitreal injection of the pharmaceutical Lucentis was performed in an operating room, the dosage was 0.5 mg (0.05 ml). In the case of the combined treatment the injection was performed 10 days prior the laser coagulation.
Results. The effectiveness of the treatment was evaluated according to OCT data (follow-up function was used) and by visometry at 5 days and 1 month after the treatment.
The dynamics of the central acuity of vision of the DME patients is presented in Figure 1.
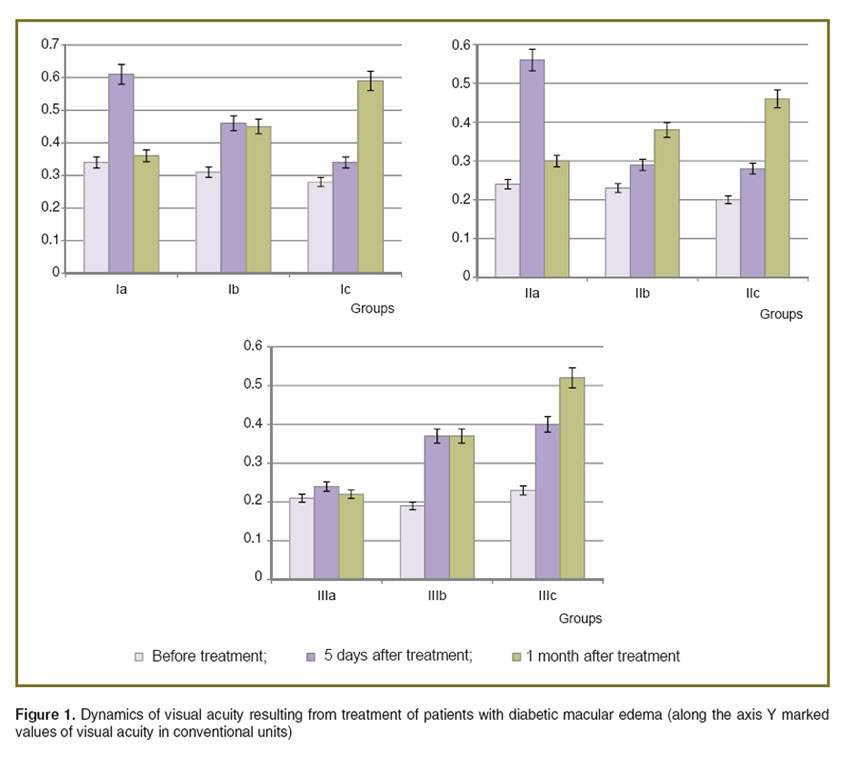 Figure 1. Dynamics of visual acuity resulting from treatment of patients with diabetic macular edema (along the axis Y marked values of visual acuity in conventional units) Figure 1. Dynamics of visual acuity resulting from treatment of patients with diabetic macular edema (along the axis Y marked values of visual acuity in conventional units)
|
The dynamics of retinal thickness in the result of the treatment of DME patients is presented in Figure 2.
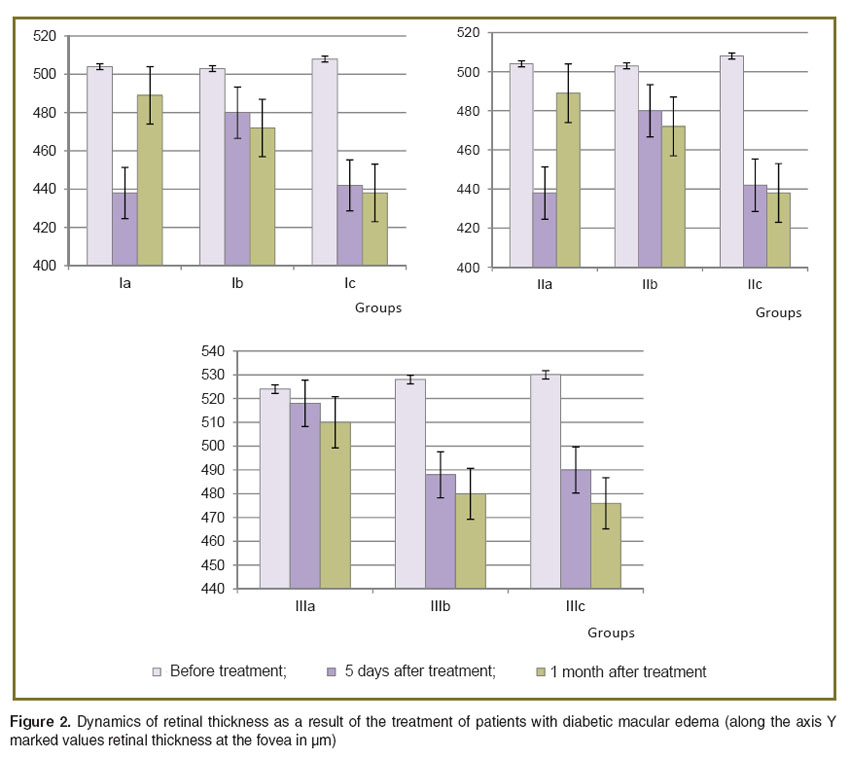 Figure 2. Dynamics of retinal thickness as a result of the treatment of patients with diabetic macular edema (along the axis Y marked values retinal thickness at the fovea in µm) Figure 2. Dynamics of retinal thickness as a result of the treatment of patients with diabetic macular edema (along the axis Y marked values retinal thickness at the fovea in µm)
|
Analysis of the data provides evidence that the effect of intravitreal injection of Lucentis was considerable, but unstable in all groups. The OCT macula data show the decrease in retinal edema after Lucentis injection; however this effect only lasted for 1 month. The use of Lucentis in cases of DME which had lasted for more than 3 months had little effect even at early stages of observation. The greatest effect of treatment across all groups, according to the OCT and visometry, was observed after the combined treatment.
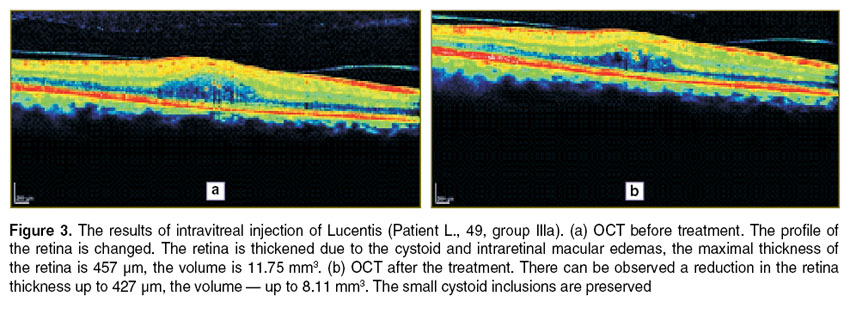
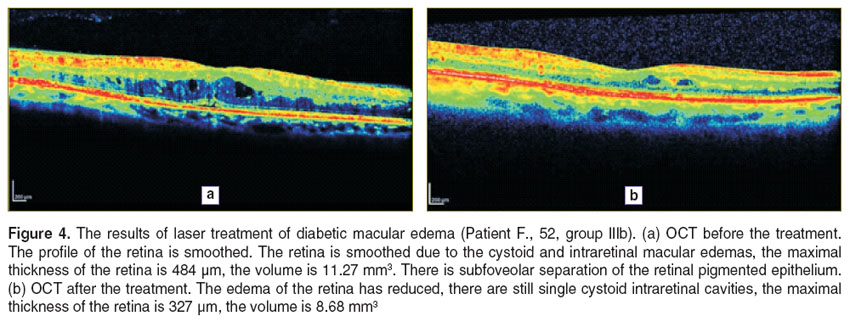
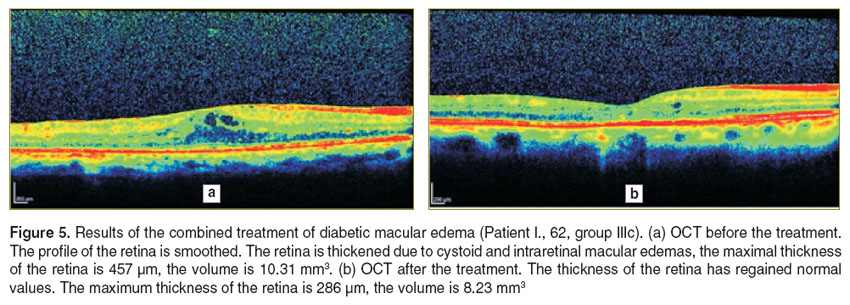
In Figures 3–5 illustrate the OCT dynamics for the different types of treatment of DME patients in whom the disease had already been present for more than 3 months. For a better perception of the image is transformed into pseudocolor where areas with a high degree of light reflection corresponding to the red and yellow, middle — green, blue and optically transparent — black.
Discussion. The effect of intravitreal injection of Lucentis was considerable, but only short-term in all the groups. This can be explained by the fact that Lucentis prevents the binding of all isoforms of VEGF-A with its receptors on the surface of endothelium cells (VEGR1 and VEGR2); however, according to the analysis of the data from pharmacokinetics and the elimination of ranibizumab from the plasma of the patients who were administered with a 0.5 mg dose of the pharmaceutical [13], the average half-life of ranibizumab from the vitreous body is about 9 days, besides which, Lucentis does not prevent the further formation of isoforms of the vascular endothelium growth factor.
After panretinal laser coagulation, there is a reliable and long-term normalization of the levels of the growth factors of the newly formed vessels [14]; however, in a number of cases macular edema was not only preserved, but increased. Among the possible reasons for such a progression of the macular edema could be an impairment of the retinal circulation as a result of acute hyperemia and the reaction of the labile prifoveolar capillaries to post-coagulation inflammation.
The best results of the treatment were achieved amongst those patients who underwent the combined treatment. The injections of angiogenic inhibitors before the laser treatment allowed completion of the stage of panretinal coagulation due to the reduction of retinal thickness and the clearing of the vessels of fibrovascular tissues.
Conclusions. OCT is an objective, highly informative method for evaluation of the effectiveness of DME treatment.According to OCT of the retina, when DME has been present for less than 1 month, the most effective treatment is a combined treatment involving laser coagulation and the injection of angiogenesis inhibitors. When DME has been present more than 1 month, laser and combined treatment have practically the same effectiveness according to our OCT data. When the treatment was completed, the index of visual acuity in all groups was highest among the patients who underwent the combined treatment.
Research Funding and Conflict of Interest. The study was not funded by any sources, and there are no conflicts of interest related to the present study.
References
- Shaw J.E., Sicree R.A., Zimmet P.Z. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract 2010; 87(1): 4–14, http://dx.doi.org/10.1016/j.diabres.2009.10.007.
- Royal College of Ophthalmologists. Guidelines for the management of diabetic retinopathy. London: Royal College of Ophthalmologists; 2005.
- Fong D.S., Ferris F.L.3rd., Davis M.D., Chew E.Y. Causes of severe visual loss in the early treatment diabetic retinopathy study: ETDRS report no.24. Early Treatment Diabetic Retinopathy Study Research Group. Am J Ophthalmol 1999; 127(2): 137–141, http://dx.doi.org/10.1016/S0002-9394(98)00309-2.
- Baskin D.E. Optical coherence tomography in diabetic macular edema. Curr Opin Ophthalmol 2010; 21(3): 172–177, http://dx.doi.org/10.1097/ICU.0b013e32833866ae.
- Davis M.D., Bressler S.B., Aiello L.P., Bressler N.M., Browning D.J., Flaxel C.J., Fong D.S., Foster W.J., Glassman A.R., Hartnett M.E.R., Kollman C., Li H.K., Qin H., Scott I.U.; Diabetic Retinopathy Clinical Research Network Study Group. Comparison of time-domain OCT and fundus photographic assessments of retinal thickening in eyes with diabetic macular edema. Invest Ophthalmol Vis Sci 2008; 49(5): 1745–1752, http://dx.doi.org/10.1167/iovs.07-1257.
- Yeung L., Lima V.C., Garcia P., Landa G., Rosen R.B. Correlation between spectral domain optical coherence tomography findings and fluorescein angiography patterns in diabetic macular edema. Ophthalmol 2009; 116(6): 1158–1167, http://dx.doi.org/10.1016/j.ophtha.2008.12.063.
- Jittpoonkuson T., Garcia P.M., Rosen R.B. Correlation between fluorescein angiography and spectral-domain optical coherence tomography in the diagnosis of cystoid macular edema. Br J Ophthalmol 2010 Sep; 94(9): 1197–1200, http://dx.doi.org/10.1136/bjo.2009.170589.
- The Royal College of Ophthalmologists. Diabetic retinopathy guidelines (December 2012). London: The Royal College of Ophthalmologists; 2012. Available: http://www.icoph.org/dynamic/attachments/taskforce_documents/2012-sci-267_diabetic_retinopathy_guidelines_december_2012.pdf.
- American Academy of Ophthalmology Retina Panel. Preferred practice pattern guidelines. Diabetic retinopathy. San Francisco, CA: American Academy of Ophthalmology; 2008.
- Beck R.W., Edwards A.R., Aiello L.P., Bressler N.M., Ferris F., Glassman A.R., Hartnett E., Ip M.S., Kim J.E., Kollman C. Three-year follow-up of a randomized trial comparing focal/grid photocoagulation and intravitreal triamcinolone for diabetic macular edema. Arch Ophthalmol 2009 127(3): 245–251, http://dx.doi.org/10.1001/archophthalmol.2008.610.
- Bandello F., Cunha-Vaz J., Chong N.V., Lang G.E., Massin P., Mitchell P., Porta M, Prünte C., Schlingemann R., Schmidt-Erfurth U. New approaches for the treatment of diabetic macular oedema: recommendations by an expert panel. Eye 2012; 26(4): 485–493, http://dx.doi.org/10.1038/eye.2011.337.
- Ford J.A., Lois N., Royle P., Clar Ch., Shyangdan D., Waugh N. Current treatments in diabetic macular oedema: systematic review and meta-analysis. BMJ Open 2013; 3: e002269, http://dx.doi.org/10.1136/bmjopen-2012-002269.
- Khonencov V.I., Klimontov V.V., Chernich V.V., Tyan N.V. Anti-VEGF agents in the treatment of diabetic macular edema. Diabetes mellitus 2013; 4, http://dx.doi.org/10.14341/DM2013478-84.
- Stahl A., Connor K.M., Sapieha P., Chen J., Dennison R.J., Krah N.M., Seaward M.R., Willett K.L., Aderman C.M., Guerin K.I., Hua J., Löfqvist C., Hellström A., Smith L.E. The mouse retina as an angiogenesis model. Invest Ophthalmol Vis Sci 2010; 51(6): 2813–2826, http://dx.doi.org/10.1167/iovs.10-5176.