Optical Coherence Tomography for Non-Invasive ex vivo Investigations in Dental Medicine — a Joint Group Experience (Review)
This review emphasizes the current knowledge related to optical coherence tomography (OCT) as a non-invasive diagnostic tool to perform ex vivo and showing great potential for in vivo structural imaging of features in the oral cavity. OCT technology can generate high-resolution cross-sectional and en-face images of the internal architecture of the investigated sample (2–3 mm in depth). To this goal, en-face time domain OCT (TD-OCT) and spectral domain OCT (SD-OCT) were employed. Topics included in this review refer to OCT non-destructive evaluations of: dental abfraction and attrition, material defects and micro-leakages at the tooth-filling interface, temporal-mandibular joint disc, quality of bracket bonding on dental hard tissue, prosthetic restorations and micro-leakages at prosthetic interfaces, root canals, presence or absence of apical micro-leakages, and osteo-integration of dental implants and of bone grafting materials. OCT revealed internal features of the material investigated with greater sensitivity than current diagnostic methods. We put our research in context with others’ results but the review reflects primarily our joint group experience and it presents images collected with our OCT systems only. The studies demonstrate the viability of OCT as a useful tool in dental medicine practice, as well as in research. Being completely non-invasive, OCT can be extended to soft tissue. Both TD and SD implementations prove the unique capabilities of OCT. For handheld scanning devices it is expected that the swept source principle (as one of the SD possibilities) will prevail, due to its high speed that allows for the reduction of distorting effects caused by the involuntary movements of the hand and of the patient. For high transversal resolution investigations, especially in more research oriented studies, it is expected that en-face TD-OCT will continue to coexist with SD-OCT methods, offering additionally a low cost quick provision of en-face view and compatibility with dynamic focus. Dynamic focus, that is the simultaneous adjustment of focus and coherence gate in depth is incompatible with SD-OCT methods and require repetitions of acquisitions under different focus in order to improve the transversal resolution, or more complex heads with division of the optical path in the object arm along different focus adjustments. In this respect, en-face TD-OCT provides a lower cost alternative to high transversal resolution of static samples.
We have shown that complementary studies are possible embracing OCT with more traditional methods, such as confocal microscopy and microCT. Combination of principles is expected to evolve due to their limitations when considered separately.
OCT systems. In the past decades, optical coherence tomography (OCT) evolved to become a powerful technique for imaging of transparent and translucent structures [1–3]. OCT is based on low-coherence interferometry (LCI), which can achieve micron-scale depth resolved quantification. The first LCI application in the biomedical optics involved the measurement of the eye length [4]. An LCI system is generally based on a two-beam interferometer. Application of such an interferometer to deliver A-scans was facilitated by a technical advantage: when moving the mirror in the reference path of the interferometer, not only is the depth scanned, but a carrier is also generated. The carrier frequency shift is Doppler shifted due to the longitudinal scanner itself. Adding lateral or angular scanning of the optical beam across the target, thin section slices can be achieved non-touch and with high axial resolution [5].
En-face time domain OCT (TD-OCT). Collecting many A-scans from various adjacent transverse positions generates B-scan images. The lines in the raster generated correspond to A-scans and are oriented along the depth coordinate. B-scan images can also be produced from T-scans at different depths. In the case of these T-scans, the transversal scanner produces the fast lines in the image [6–8], by controlling either the transverse scanner along the X-coordinate (horizontal), along the Y-coordinate (vertical) or along a polar angle q, while the other two scanners remain fixed. This procedure has a clear advantage in comparison with the A-scan based B-scan procedure as it allows for the production of OCT transverse (or en-face) images for a fixed reference path; such images are called C-scans.
For TD-OCT the path length of the reference arm is scanned in time. Interference (i.e., the series of dark and bright fringes) is only achieved when the optical path difference (OPD) lies within the coherence length of the optical source. The envelope of this modulation changes as the OPD is varied; the peak of the envelope corresponds to the path length matching [9–11].
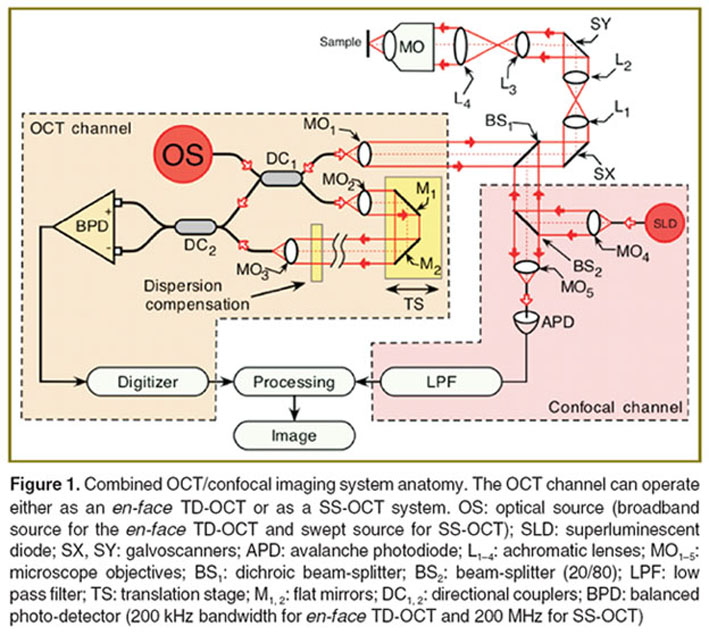
The schematic diagram of the en-face TD-OCT system used in our studies is depicted in Figure 1. The optical source (OS) employed in this regime of operation was a pigtailed superluminescent diode (SLD) emitting at 1300 nm and with a spectral bandwidths of 65 nm. This determines an OCT longitudinal resolution of 8.6 μm in teeth. Depending on the ratio between the focal lengths of lenses L3 and L4, the lateral resolution and maximum achievable lateral size of the en-face images can be tweaked. We used two configurations:
(i) Low numerical aperture (NA) interface optics, which allows 1 cm lateral image size.
(ii) High NA interface optics with a maximum 1 mm lateral image size.
In addition to the OCT channel, the imaging system is equipped with a confocal channel using as light source a second SLD emitting at 970 nm. This channel was used in some of the investigations to guide the adjustment of the sample as well as to provide some complementary reflectivity measurements.
Light from the OS is injected into the system via a first directional coupler (DC1), which splits the light toward the two arms of the interferometer, the probing and reference arms respectively. The probing beam is reflected by the dichroic beam-splitter (BS1) and then sent via the galvanometer scanners SX and SY towards the sample. BS1 is a hot mirror that reflects light of wavelengths longer than 1 μm. Two telescopes incorporated between these elements conveniently alter the diameter of the beam to match the aperture of different elements in the probing path and convey a probing beam of 8 mm in diameter through the microscope objective (MO) pupil plane when a high resolution mode is required. The two transverse scanners SX and SY are separated here using telescopes to project a flat wave front on the target under high NA. Lenses L1, L2, and L4 have focal lengths of 7.5 cm, while lens L3 has a focal length of 3 cm. The MO is a scan lens (focal length 1.8 cm) to prevent image degradation and distortion during scanning. Hence a lateral resolution of around 2 μm was experimentally measured in the confocal channel and better than 5 μm in the OCT channel. Light backscattered by the sample passes a second time through the object arm and is guided via the DC1 toward the second single mode directional coupler (DC2), where it interferes with the light coming from the reference arm. Both output fibers from the second coupler are connected to two pin photo-detectors in a balanced photo-detection (BPD) unit. After digitization, the OCT signal is rectified and low-pass filtered in the processing block.
The confocal channel operates at a different wavelength than that of the OCT, to allow the utilization of a high gain silicon avalanche photodiode (APD). The light from the SLD at 970 nm is collimated by a MO4 and reflected by a beam-splitter (BS2) (20% reflection) toward BS1. Light at 970 nm is transmitted via BS1 and BS2 toward the APD. The photo-detected signal is amplified and low-pass filtered in LPF. A computer-driven translation stage (TS) is used to alter the reference path length in order to acquire C-scan image frames from different depths and thus to provide depth scanning in the B-scan regime.
The scanning procedure is similar to that used in any confocal microscope, where the fast scanning is en-face (line rate, using the galvanometer scanner SX) and the frame scanning is much slower (at the frame rate, using the galvanometer scanner SY) [12]. The frame grabber in Figure 1 is controlled by signals from the generators driving SX and SY galvanometer scanners. The SX galvanometer scanner is driven with a ramp at 500 Hz and the SY galvanometer scanner with a ramp at 2 Hz. In this way, an en-face image, in the plane (X, Y) is generated at constant depth. The next en-face image at a new depth is then generated by moving the TS in the reference arm of the interferometer and by repeating the (X, Y) scan. Ideally, the depth interval between successive frames should be much smaller than the system resolution in depth, and the depth change is applied only after the entire en-face image has been collected. However, in practice, to speed up the acquisition, the TS was moved continuously.
To construct B-scan images, no signal is applied to the frame scanner, while the line scanner is driven with the same signal as in the C-scanning regime, and the TS is moved along the optical axis of the reference beam. In this case, the frame grabber is controlled by signals from the generator driving the SX (or the SY) with a ramp at 500 Hz, and TS is moved over the depth range required in 0.5 s. Thus, an OCT cross section image is produced either in the plane (X, Z) or (Y, Z).
In the images presented in this manuscript, no other phase modulation was employed apart from that introduced by the galvanometer scanner [6, 8] determining the line in the raster.
In the low NA configuration, L3 and L4 have been removed, only a single optical component being placed between the SX, SY and the sample (MO, focal length 4 cm) allowing a larger lateral size image. Consequently, the transversal resolution was reduced to 15 μm.
Spectral domain OCT (SD-OCT). In SD-OCT, the spectrum at the output of the LCI is measured. A Fourier transformation of the acquired spectrum delivers an A-scan, without any movement in the reference arm [13–16]. Due to the fact that all depths are obtained in one measurement, the speed of producing B-scan images improves dramatically, as well as the signal-to-noise ratio in comparison to TD-OCT. As drawbacks, SD-OCT cannot provide a faster production of en-face images or axial ranges longer than those achievable in TD-OCT. SD-OCT can be implemented in two ways: swept source OCT (SS-OCT) and camera based, or spectrometer based (Sp-OCT). In some papers, one or both of these methods are referred to as Fourier domain OCT methods. Both types of methods have been employed in instruments we have assembled, we show here results obtained using the SS-OCT principle only.
A SS-OCT instrument was assembled, with a diagram similar to that used for TD-OCT (See Figure 1). In this case the OS is a fast SS and the photo-detector is changed to a faster one (over 200 MHz instead of 400 kHz). The SS is from Axsun Technologies Ltd. (central wavelength 1060 nm, sweeping range of 106 nm (measured at 10 dB), average output power of 16 mW, sweeping rate 100 kHz). A depth resolution determined by the SS tuning range of 12 μm in air was experimentally measured. The SS-OCT system was used in a low NA configuration; hence a lateral resolution of ~14 μm was experimentally measured. The optical power on the sample is 3.6 mW. The DC2 output signals are sent to a fast balanced photo-detector BPD (model PDB460C, bandwidth 200 MHz, Thorlabs, Inc., USA). A 12-bit analog-to-digital acquisition card digitized the output of the photo-detector (Alazartech ATS9350, Canada), while an “in-house” LabVIEW (National Instruments, USA) created software is used to produce, display, and record the images. The lateral size of the 3D images, determined by the amplitude of the voltages applied to the galvoscanners and the focal length of MO, is 4.4×4.4 mm, while their axial size, determined by the SS and the digitizer is 3.7 mm. The system is able to produce 500×640 pixels B-scan images (cross-sectional images of the sample) at a frame rate of 100 Hz. 3D reconstructed images of 500×500×640 pixels could then be produced. The inspection of the volume can be performed either along B-scans or C-scans. As 500 A-scans are used to construct each B-scan image, given the sweeping speed of the SS, a number of 100 B-scans are acquired per second. Hence, in order to acquire data to reconstruct a 3D volume made of 500 B-scans, 2.5 s are needed.
A Sp-OCT was also assembled, operated at 840 nm using an Aviva camera (Atmel, USA) at 29 kHz line rate. It was used in a single study here, to produce B-scans only, therefore its diagram is not shown. Interested readers can find more data in [17].
The various OCT applications in dentistry tested by our research group will be presented, considering each investigated structure at a time. The imaging systems, as presented above have been used at different stages of our research activity, in line with the OCT progress from TD-OCT towards SD-OCT methods. Compared with TD-OCT, SD-OCT methods present the advantage of increased phase stability for functional imaging. However, SD-OCT methods exhibit three main disadvantages: decay of sensitivity with OPD, impossibility to move the focus to the depth investigated while scanning and symmetric (ghost) images if the OPD=0 position crosses the object volume, problem known as mirror terms. The impossibility of focusing at selected depths renders the technology unsuitable to high transversal resolution microscopy, where TD-OCT is favored. If minute details are to be identified in the sample, then TD-OCT is better. In addition, en-face TD-OCT can deliver an en-face image direct, while even the fastest SD-OCT systems known today still need several seconds to acquire all data volume and produce the en-face image by software means. In case large size images are to be generated from soft moving tissue, then SD-OCT methods should be used for its higher speed.
Clinical results. In dentistry, current scientific literature shows that OCT has been successfully used for acquiring images of: dental biofilm [18, 19], attrited teeth [20], enamel erosion [21], dentin structures [22–25], vertical root fractures [26–27], and incipient carious lesions [28–46]. It was also used for the evaluation of severity in advanced carious lesions [47] and re-mineralization of root caries [48], dentin re-mineralization [49–50], lesion progress in root caries [51], for quantification of re-mineralization [52–57], as well as for determining the efficiency of different agents in the inhibition of demineralization [52, 58–64]. Additionally, several research groups have demonstrated that OCT is capable to evaluate the oral mucosa [65–67], the micro-leakages and internal defects of composite restorations [68–81], dental sealants [82–84] and endodontic fillings, the root internal structure [85, 86], the dental implant-abutment interface [87], and dental adhesives [88]. OCT is able to identify early signs of inflammation, unlikely to be detected by clinical examination. OCT imaging offers the exciting potential to detect peri-implantitis before significant osseous destruction occurs [89]. OCT has also been employed for periodontal diagnosis [90], for evaluating the integrity of dental prosthesis, their quality [91], their marginal fitting [30, 47, 92, 93] and their adhesion to the tooth structure [94, 95], for monitoring the periodontal ligament changes induced by orthodontic forces [96, 97], and orthodontic interfaces [98–101].
Hard dental tissue (noncarious lesions)
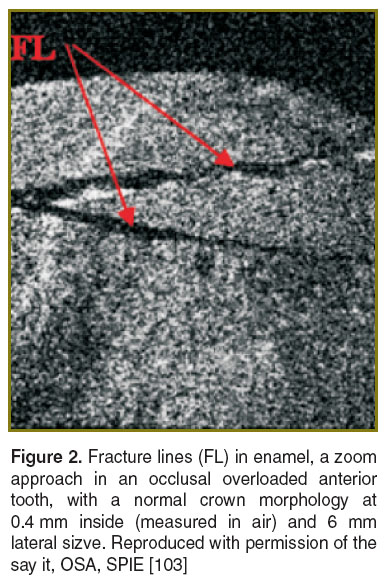
Occlusal overload. Occlusal overload represents a major concern to dentists because of its undesired consequences, which include excessive tooth wear (pathological attrition and abfractions), dental crown and/or root fracture, failure of dental restorations, and temporo-mandibular disorders. An early diagnosis is essential in such cases. Our group has demonstrated that OCT represents a promising, non-invasive alternative technique for early detection and monitoring of occlusal overload in bruxing patients [102–104]. En-face TD-OCT and fluorescence microscopy were used to investigate the wear of anterior teeth derived from young patients with active bruxism (strong, unconscious, and rhythmic grinding and/or clenching of teeth during the day or during the sleep [105], mainly caused by high levels of emotional stress and by occlusal interferences during protrusive or laterotrusive mandibular movements) [103]. The teeth presented first-degree pathological wear established through a tooth wear index (TWI), which was proposed by Smith and Knight in 1984 in order to score the wear of all four visible dental surfaces [106]. In our study the TWI score equals 1 (showing loss of enamel surface characteristics without exposing dentine, with a minimal loss of contour). The combination of information collected by OCT and fluorescence microscopy revealed a characteristic pattern of enamel cracks that reached the tooth surface.
En-face TD-OCT was also used to investigate extracted anterior teeth with a normal crown morphology (without pathological attrition), which derived both from patients with active first-degree bruxism diagnosed by using BiteStrip devices and from subjects without parafunction [103]. The teeth from non-bruxing patients revealed a homogenous structure of the superficial enamel on the en-face TD-OCT images. Despite the normal crown morphology, the teeth extracted from patients with first-degree bruxism showed signs of enamel damage in the OCT images. This consisted of a characteristic pattern of cracks, which did not reach the tooth surface (Figure 2).
Abfraction and attrition. The evolution of the pathological dental wear over time is essential for the prognosis of teeth and for the initiation of the most suitable therapeutic steps. Monitoring these lesions helps to determine whether a particular phenomenon is progressive or not.
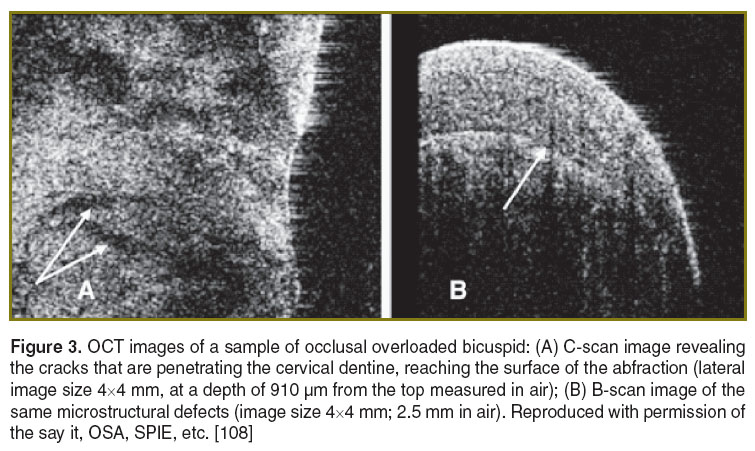
Our research team demonstrated microstructural characterization of abfraction by en-face TD-OCT [107, 108]. The investigation of bicuspids with normal crown morphology revealed a homogenous structure of the buccal cervical enamel. Occlusal-overloaded bicuspids (derived from patients with active bruxism) were imaged by the system in Figure 1, which produced constant depth OCT images (C-scans) as well as cross cross-section OCT images (B-scans). These images revealed the wedge-shape loss of cervical enamel and the damage of the underlying dentin. The high-occlusal forces produced a characteristic pattern of large cracks, which reached the tooth surface (Figure 3).
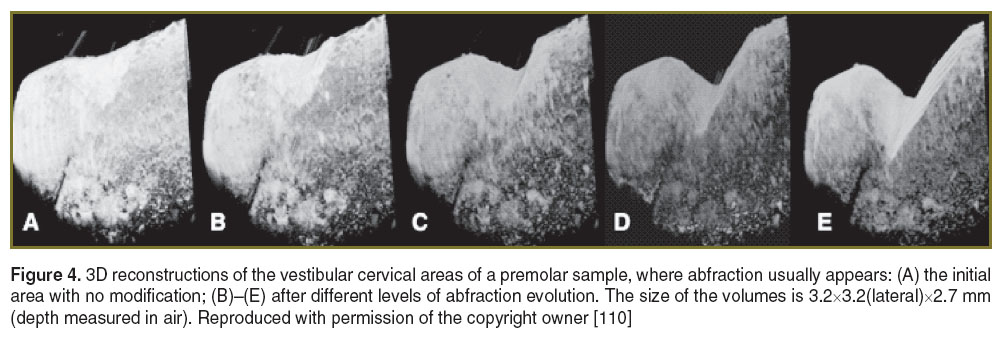
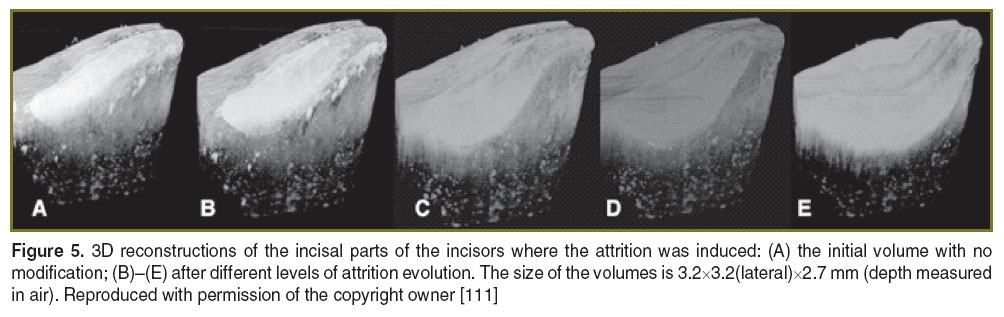
In other studies from our group [109–111], artificially induced defects (attrition and abfractions) in teeth were similar to those observed in the clinic (scored by the TWI in terms of enamel and dentine loss). The loss of dental hard tissue can be qualitatively evaluated by inspecting either two-dimensional images or 3D reconstruction of volumes. Our study has shown that a more accurate figure on evaluation of such processes is provided by 3D reconstructions, as individual B-scans may show less loss. When the 3D reconstruction was considered, the maximum depths of the abfractions were larger than the values measured in some individual B-scan images, leading to: 0.41 mm (Figure 4 (C)), 0.98 mm (Figure 4 (D)), and 1.173 mm (Figure 4 (E)). Using the 3D reconstructions of the attritions, again, the maximum depth values obtained were different from the values measured on the B-scan images: 0.33 mm (Figure 5 (B)), 0.67 mm (Figure 5 (C)), 0.83 mm (Figure 5 (D)), and 1.083 mm (Figure 5 (E)). The OCT system used for this study presented a minimum volume detectable change in the sample of ~2352 μm3.
The amount of hard dental tissue lost, as well as the evolution of such loss is important. Providing these two pieces of information constitutes a useful guidance for the clinical evaluation of abfraction and attrition.
Another major advantage of this technology is provided by the speed of acquisition, more than 100 times faster than the TD-OCT used in our previous studies [102–104].The method is equally applicable to faster systems working at over 1-MHz line rate [112, 113]. A high-speed SS-OCT may therefore prove a more suitable clinical tool to quantify abfraction and attrition.
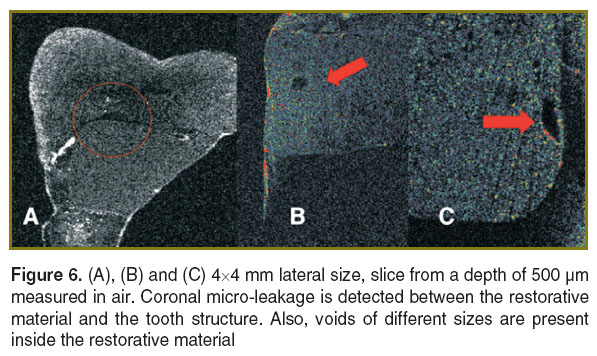
Dental fillings. En-face TD-OCT shows promise in the examination of structural quality of these restorations. In several of our preliminary studies, amalgam [114], composite resin [115–118], and compomer [119] have been used to restore teeth. The amalgam (by virtue of its metallic composition) completely obscures the tooth interior beneath it in any OCT image. However, the other two materials, by virtue of their small absorption coefficients, allow for the visualization of internal landmarks such as the dental enamel junction in the tomogram (Figure 6), obtained with the system in Figure 1 [120].
OCT is limited in the capability of separating the interfaces between the adhesive layer, the dental tissue and the resin; the adhesive layer cannot sometimes be told apart from air gaps. The issue is that interfaces between different materials are detected in OCT based on the difference between their indices of refraction [30], and the tooth adhesives present a quite low refractive index. Therefore, in order to better visualize such challenging interfaces, we proposed to employ materials that dope the adhesive to strengthen the backscattered light [121, 122]. In a recent study [123], the procedure of enhancing the contrast in the OCT image by applying an optimum concentration of zirconia to the adhesive layer was evaluated. This procedure allowed better visualization of the integrity of the adhesive fillings, by enhancing the interfaces between the adhesive and the tooth structures and between the adhesive and the composite resin.
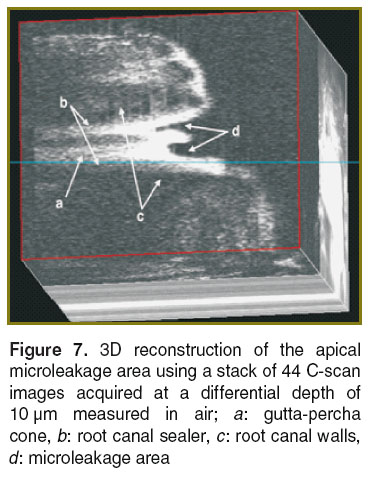
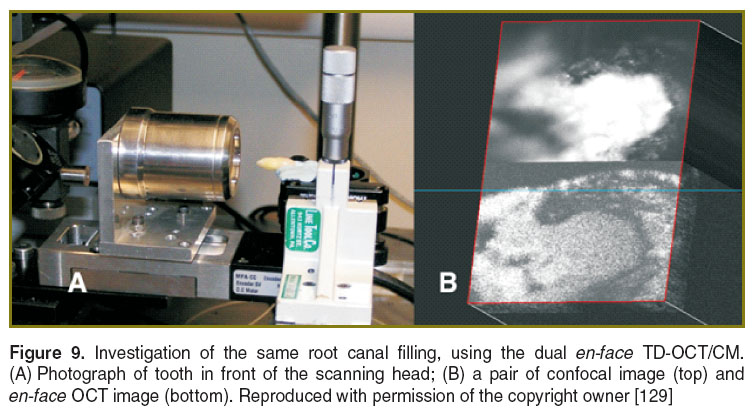
Endodontic treatment. The quality of endodontic treatments and root canal fillings were investigated with en-face TD-OCT technology [124–129]. It was possible to identify areas of apical micro-leakage between the filling material of the root canal space, the gutta-percha cones, and the root canal walls (Figures 7 and 8). For a better assessment of the quality of the endodontic treatment, the system equipped with a second channel, confocal, for guidance (as shown in Figure 1) was employed. This set-up provides both dual imaging and magnified view. The confocal image aids guidance and allows for focus adjustment in the OCT investigation. In order to obtain the images, sections up to a depth of 2 mm were scanned (Figure 9), thus quantifying the level at which defects appeared within the filling material or between the filling material and the gutta-percha material. During the examination, it has been evidenced that in some samples, defects were present in all sections to a full depth of 2 mm, while in others defects were observed in fewer layers. This observation allowed for a quantitative statistical analysis based on the number of sections in which defects were present.
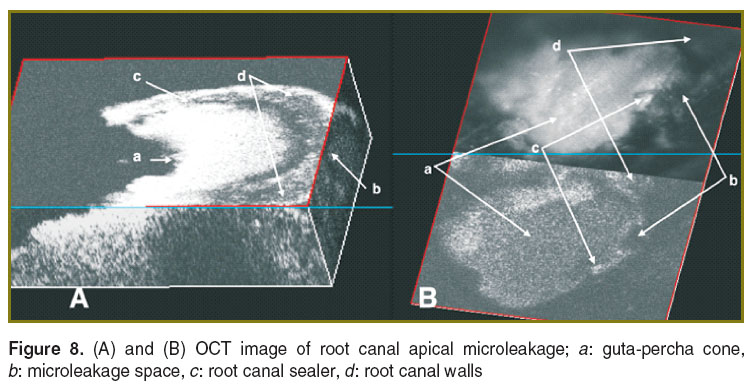 Figure 8. (A) and (B) OCT image of root canal apical microleakage; a: guta-percha cone, b: microleakage space, c: root canal sealer, d: root canal walls Figure 8. (A) and (B) OCT image of root canal apical microleakage; a: guta-percha cone, b: microleakage space, c: root canal sealer, d: root canal walls
|
In a different study [130], we employed OCT to investigate the fitting and the gap width between fiber posts, adhesive luting cement and root canal wall. The results obtained have proven the importance of assessing the interface quality of the fiber post, adhesive luting cement and root canal wall after each fiber post luting process.
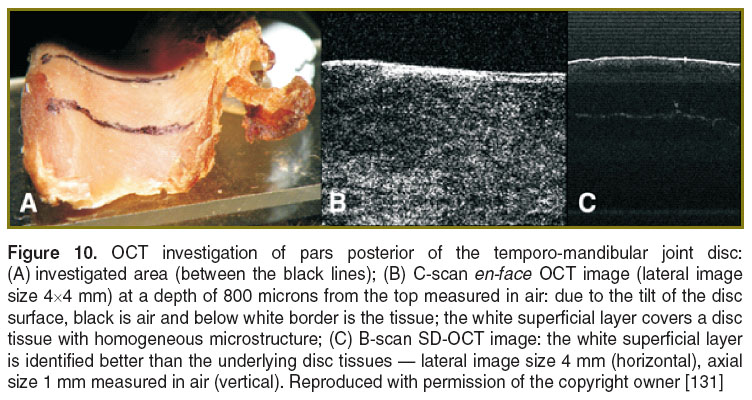
Temporo-mandibular joint disc. In a preliminary study [131], we revealed the micro-structural characterization of the temporo-mandibular disc by using both en-face TD-OCT and SD-OCT. 8 human temporo-mandibular joint discs were harvested from dead subjects, having less than 40 years of age; the samples were then conserved in formalin. They presented normal morphology, with thicker posterior pars (2.6 mm on the average) and thinner intermedia pars (1 mm on the average). The OCT investigation of the healthy temporo-mandibular joint discs revealed their homogeneous microstructure, covered by a white superficial layer (Figure 10). The longer wavelength of the TD-OCT assembled offers a larger penetration depth (2.5 mm in air) than that achievable in the Sp-OCT system (1.5 mm), which is important for the analysis of the pars posterior, while the Sp-OCT proved to be much faster.
The longer wavelength of the TD-OCT allowed better identification of the homogenous microstructure of the thicker posterior pars on C-scan images; a continuous white superficial layer of a proteoglycan could also be visualized at 1300 nm. The detailed observation was allowed by the TD-OCT method, compatible with dynamic focus.
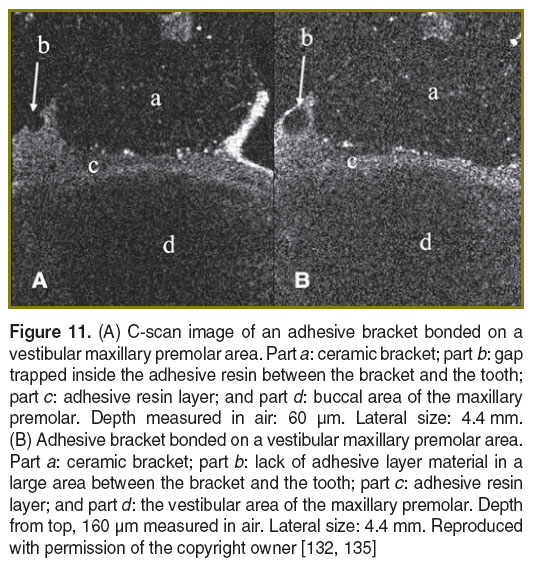
Orthodontics. The en-face TD-OCT system shown in Figure 1 was also used in our studies to evaluate the connection between the bracket and the tooth structure [132]. Orthodontic attachments bonding strength cannot be measured with OCT; however, identifying and visualizing the voids in the composite can provide some indication on the quality of the bonding [133, 134]. OCT investigations provide information on the micro-leakage of the bracket’s bonding; in Figure 11 (A), several gaps can be seen along the bracket base. Also, it was possible to identify a lack of adhesive material on the side of the bracket (Figure 11 (B)). Although this work was restricted so far to ex vivo investigations [135, 136], the tooth-bracket interfaces could also be imaged in vivo by assembling special hand held probes as described in [137].
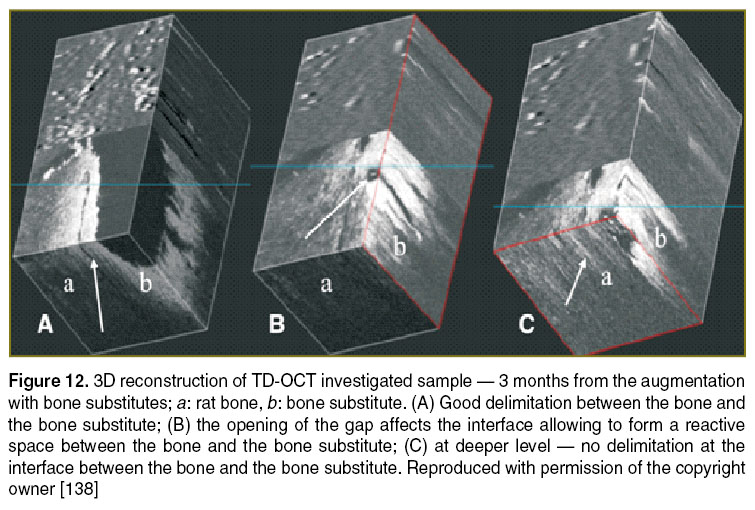
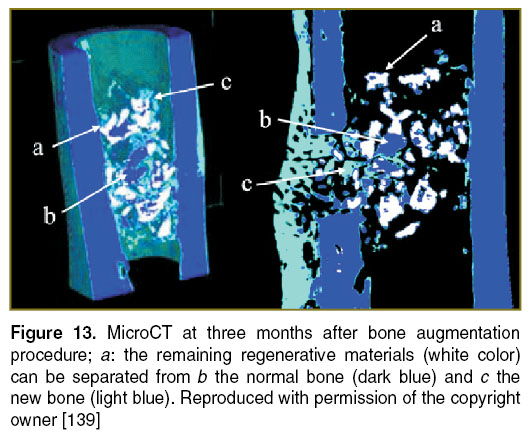
Implantology. Bone grafting is a commonly performed surgical procedure for bone regeneration in a variety of orthopaedic and maxillofacial procedures. There are several aspects that require attention when implementing this type of procedures, such as consideration of materials used in bone regeneration, quality control methods, adjustment of the initial bone regenerative scaffold, monitoring of their osteoconduction and osteo-integration period, and biomedical evaluation of the new regenerated bone. It would be valuable to extend the OCT evaluation of the bone grafting material/bone interface to a wide area of hard tissue augmentation procedures. The quality of bone grafting was evaluated by using TD-OCT (Figure 12) and validated by using microCT (Figure 13). Based on these studies, it becomes easier to extend OCT to in vivo evaluations to provide qualitative and quantitative data on the bone augmentation procedures [138–140].
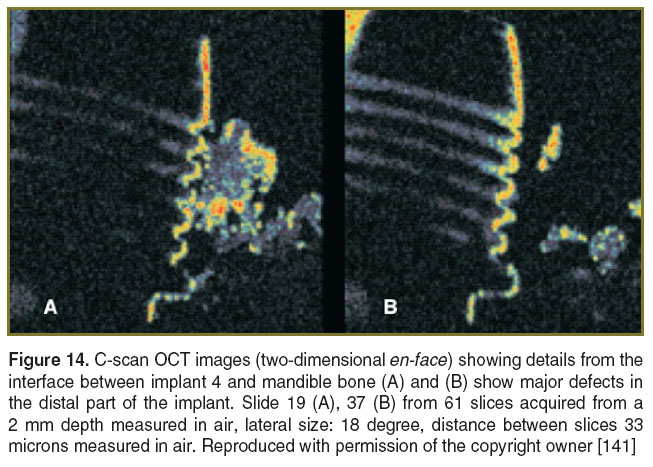
The quality of the implant insertion can be investigated by using the analysis of the interface between the implant and the bone. We have initiated two studies on the numerical simulation and tensional stamps. Both have demonstrated that en-face TD-OCT is able to evaluate the morphology of interfaces between the implant and the bone [141, 142]. Both C-scan and B-scan OCT images were collected. 3D analysis wasmade possible by acquiring a number of 30 to 100 C-scans that were used post-acquisition to explore the volume of the tissue around these interfaces. The authors chose to evaluate the implant-supported restorations on two implants connected with conjunction bars and the implant supported restorations on four implants connected with a bar.
For the model development we used the ANSYS Computer-Aided Engineering (CAE) software. The ANSYS CAE software program was used in conjunction with a 3D Computer-Aided Design (CAD) to simulate the behavior of mechanical parts under thermal/structural loading conditions. ANSYS automated Finite Element Analysis (FEA) technologies from ANSYS, Inc. (USA) were used to generate the results listed in this report. Numerical simulation investigations generated specific charts that allowed for the quantitative evaluation of the tensions around the implants and in the bone structure. The micro measurement method was used to validate the results obtained using the numerical simulations. It was demonstrated that the four implants configuration with a connecting bar is better than the solution of two implants with a connecting bar because the tensions in the implant bone interfaces are lower. The most important feature is represented by the intimate contact between the implant and the bone. To investigate such aspects, it is again essential to employ a non-invasive method to predict the stability of the inserted implants. En-face TD-OCT images from different interfaces between mandible bone and the implants are shown in Figure 14. Using occlusal scanning, numerous spaces between the implant and the bone were found. These can be the source of deficient biomechanical behaviour of the implant-supported restoration. The classical investigation methods like nuclear magnetic resonance and computed tomography are invasive and they exhibit inferior resolution. Therefore these methods cannot ensure a good prognostic of the dental treatment, while OCT is capable to achieve better overall resolutions while being non-invasive.
Prosthodontics. Sequential and rapid switching between the en-face regime and the cross-section regime, specific for the en-face TD-OCT technology represents a significant advantage in the non-invasive examination of dental prostheses [120, 143–158]. Several types of such prostheses were investigated, including metal-ceramic fixed partial prostheses, metal-ceramic crowns, metal-polymer fixed partial prostheses, metal-polymer crowns, polymer and all-ceramic fixed partial prostheses, and complete dentures. The main goal has been to detect the presence or absence of material defects and micro-leakages at the prosthetic interfaces.
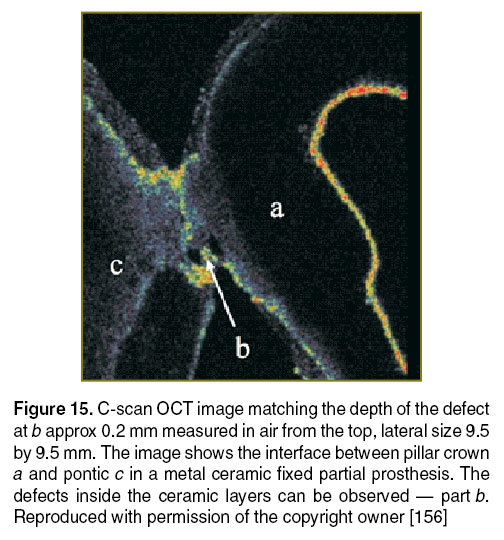
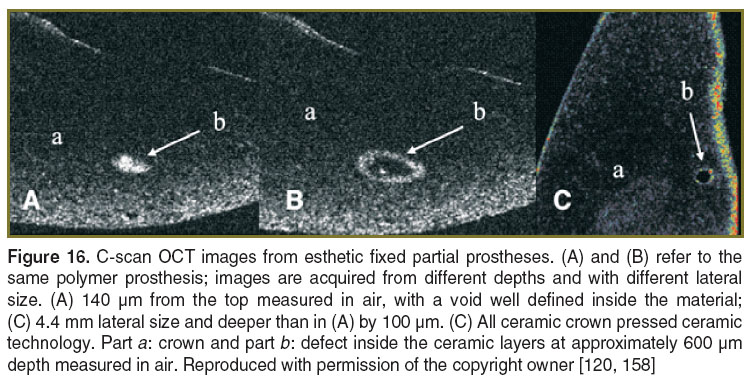
In several of the prostheses investigated by our group, defects that may cause their fracture were identified. The areas depicted in the OCT images present several small canals in the base that can be colonized in time with bacteria. Such areas can also cause the start for esthetic and functional failure of the prosthetic treatment. The defects are usually located inside the material and cannot be depicted visually or by any other conventional imagistic method. A series of these defects are illustrated in Figures 15 and 16, obtained using the en-face TD-OCT system.
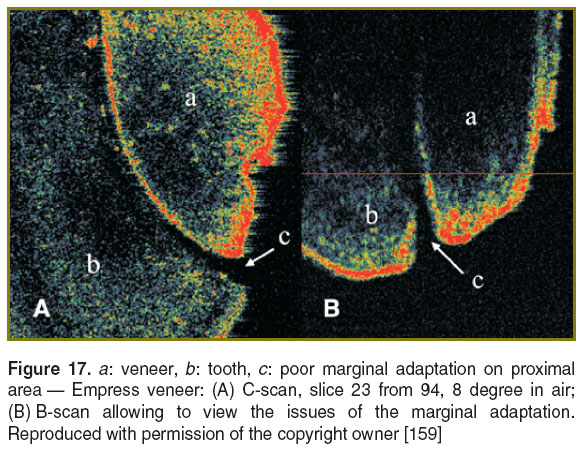
In yet another study, we have analyzed the potential of en-face TD-OCT for the investigation of all-ceramic veneers immediately after the bonding process. This evaluation can be useful for establishing the prognostic of the prosthetic treatment. 32 Empress veneers (Ivoclar Vivadent, Lichtenstein) were investigated using en-face TD-OCT. The scanning procedure was performed vestibular, oral, mesial and distal for each sample [159]. The investigation revealed poor marginal adaptation for 18 out of 32 samples. The marginal adaptation problems were identified especially in the proximal and oral areas (Figure 17).
Handheld scanning probes for OCT. Several variants of handheld scanning probes for OCT were developed in our groups, in an effort to make them as light, simple and low-cost as possible. We have shown that probes can be constructed almost entirely from off-the-shelf parts and ergonomic designs, like that shown in Figure 18 [137]. This has been used for an ex vivo study of metalo-ceramic dental prosthesis [137].
 Figure 18. Handheld scanning probe — ergonomic variant [137] Figure 18. Handheld scanning probe — ergonomic variant [137]
|
The handheld probes devised so far employ a one-dimensional galvanometer scanner (1D GS), that provide distortion-free OCT images when driven by triangular signals [160, 161]. In contrast, Micro-electro-nechanical systems (MEMS)-based handheld probes are sinusoidally driven, using resonant oscillatory mirrors; therefore they require post-processing of OCT images in order to remove distortions [162]. Progress is under way to develop two-dimensional scanning heads to provide volumetric reconstructions of the samples investigated [163].
The 1D GS-based handheld probes developed in [137] reached a mass of 0.35 kg, in comparison to MEMS-based probes, of 0.5 kg [162] and to commercially available probes of 1.5 kg (Bioptigen Envisu, USA) or 2.2 kg (Optovue iVue, USA).
A handheld probe developed in our group has already been applied to an in vivo study in an ENT Department in a clinical environment [164]. The development of handheld probes is part of our effort to extend ex vivo to in vivo clinical investigations.
Conclusions. The paper presents the experience of our multidisciplinary team in acquiring OCT images using several own assembled OCT systems. Using such systems, we investigated and assessed non-destructively hard dental structures, dental materials, dental restorations and the temporal-mandibular joint disc. The studies demonstrate the viability of OCT as a useful tool in dental medicine practice, as well as in research. Being completely non-invasive, OCT can be extended to soft tissue. It is also expected that technological progress in the most important area for OCT applications at the moment, eye imaging, will spur collateral advancement of OCT development in dentistry. For instance, decrease in the cost of handheld devices used in imaging the anterior chamber of the eye, will impact the cost of similar systems that can be applied to dentistry with a minimum of alterations. Both TD and SD implementations prove the unique capabilities of OCT. For handheld scanning devices it is expected that the swept source principle (as one of the SD possibilities) will prevail, due to its high speed that allows reduction of distorting effects caused by involuntary movements of the hand and of the patient. However, for high transversal resolution investigations, especially in more research oriented studies, it is expected that en-face TD-OCT will continue to coexist with SD-OCT methods, offering additionally a low cost quick provision of en-face view and compatibility with dynamic focus. Dynamic focus, that is the simultaneous adjustment of focus and coherence gate in depth is incompatible with SD-OCT methods and require repetitions of acquisitions under different focus in order to improve the transversal resolution, or more complex heads with division of the optical path in the object arm along different focus adjustments. In this respect, en-face TD-OCT provides a lower cost alternative for high transversal resolution of static samples.
We have shown that complementary studies are possible by combining OCT with more traditional methods, such as confocal microscopy and microCT. Combination of principles is expected to evolve in the near future due to their limitations when considered separately.
TD-OCT and SD-OCT systems were used separately in our studies, for different applications, as presented. However, research has shown improved performance achievable by combining principles of TD and SD interferometry. For the moment, implementation of combination of such principles is still expensive, but once implemented, the architectures obtained show promise in terms of simultaneous en-face imaging or in terms of extending the axial range of SD methods. They may find niche applications, first in handheld scanning devices and second in long axial catheters to be used on the nerve canals. Dentistry, as well as ophthalmology, will continue to benefit from the continuous advancement in the OCT hardware and customization of different principles to specific applications.
Acknowledgements. Some of the results described here were obtained with support from a grant of the Romanian Authority for Scientific Research, CNDI-UEFISCDI project PN-II-PT-PCCA-2011-3.2-1682 (http://3om-group-optomechatronics.ro/). The work also derived from collaboration activities within the framework of the FP 7 MPNS Action COST MP1005 “From nano to macro biomaterials (design, processing, characterization, modelling) and applications to stem cells regenerative orthopedic and dental medicine (NAMABIO)”. C. Sinescu acknowledges the support of the TE 101/2010 CNDI-UEFISCDI grant. A. Bradu and A. Podoleanu acknowledge the support of ERC 7th Framework Programme, Advanced Grant ‘COGATIMABIO’249889. A. Podoleanu also acknowledges the NIHR Biomedical Research Centre at Moorfields Eye Hospital NHS Foundation Trust, and of the UCL Institute of Ophthalmology.
Conflict of Interests. The author declares no conflict of interest.
References
- Fujimoto J.G., Brezinski M.E. Optical coherence tomography imaging. In: Biomedical photonics handbook. Vo-Dinh T. (editor). CRC Press; 2003; p. 22–24, http://dx.doi.org/10.1201/9780203008997.ch13.
- Brady D.J. Optical imaging and spectroscopy. Wiley & Sons, Inc.; 2009, http://dx.doi.org/10.1002/9780470443736.
- Optical coherence tomography. Drexler W., Fujimoto J.G. (editors). Springer Berlin Heidelberg; 2008, http://dx.doi.org/10.1007/978-3-540-77550-8.
- Fercher A.F., Roth E. Ophthalmic laser interferometry. Proc. SPIE, Optical Instrumentation for Biomedical Laser Applications 1986; 0658: 48–51, http://dx.doi.org/10.1117/12.938523.
- Huang D., Swanson E.A., Lin C.P., Schuman J.S., Stinson W.G., Chang W., Hee M.R., Flotte T., Gregory K., Puliafito C.A., Fujimoto J.G. Optical coherence tomography. Science 1991; 254(5035): 1178–1181, http://dx.doi.org/10.1126/science.1957169.
- Podoleanu A.G., Dobre G.M., Webb D.J., Jackson D.A. Coherence imaging by use of a Newton rings sampling function. Opt Lett 1996; 21(21): 1789–1791, http://dx.doi.org/10.1364/ol.21.001789.
- Podoleanu A.G., Dobre G.M., Jackson D.A. En-face coherence imaging using galvanometer scanner modulation. Opt Lett 1998; 23(3): 147–149, http://dx.doi.org/10.1364/ol.23.000147.
- Podoleanu A.G., Seeger M., Dobre G.M., Webb D.J., Jackson D.A., Fitzke F.W. Transversal and longitudinal images from the retina of the living eye using low coherence reflectometry. J Biomed Opt 1998; 3(1): 12–20, http://dx.doi.org/10.1117/1.429859.
- Fercher A.F. Optical coherence tomography — development, principles, applications. Z Med Phys 2010; 20(4): 251–276, http://dx.doi.org/10.1016/j.zemedi.2009.11.002.
- Choma M.A., Sarunic M.V., Yang C., Izatt J.A. Sensitivity advantage of swept source and Fourier domain optical coherence tomography. Opt Express 2003; 11(18): 2183–2189, http://dx.doi.org/10.1364/OE.11.002183.
- Leitgeb R., Hitzenberger C.K., Fercher A.F. Performance of Fourier domain vs. time domain optical coherence tomography. Opt Express 2003; 11(8): 889–894, http://dx.doi.org/10.1364/oe.11.000889.
- Masters B.R. Three-dimensional confocal microscopy of the human optic nerve in vivo. Opt Express 1998; 3(10): 356–359, http://dx.doi.org/10.1364/oe.3.000356.
- Park B.H., Pierce M.C., Cense B., Yun S.-H., Mujat M., Tearney G.J., Bouma B.E., de Boer J.F. Real-time fiber-based multi-functional spectral-domain optical coherence tomography at 1.3 μm. Opt Express 2005; 13(11): 3931–44, http://dx.doi.org/10.1364/opex.13.003931.
- Wang K., Ding Z., Wu T., Wang C., Meng J., Chen M., Xu L. Development of a non-uniform discrete Fourier transform based high speed spectral domain optical coherence tomography system. Opt Express 2009; 17(14): 12121–1231, http://dx.doi.org/10.1364/oe.17.012121.
- Gützinger E., Baumann B., Pircher M., Hitzenberger C.K. Polarization maintaining fiber based ultra-high resolution spectral domain polarization sensitive optical coherence tomography. Opt Express 2009; 17(25): 22704–22717, http://dx.doi.org/10.1364/oe.17.022704.
- Wang Y., Nelson J.S., Chen Z., Reiser B.J., Chuck R.S., Windeler R.S. Optimal wavelength for ultrahigh-resolution optical coherence tomography. Opt Express 2003; 11(12): 1411–1417, http://dx.doi.org/10.1364/OE.11.001411.
- Bradu A., Ma L., Bloor J.W., Podoleanu A.G. Dual optical coherence tomography/fluorescence microscopy for monitoring of Drosophila melanogaster larval heart. J Biophotonics 2009; 2(6–7): 380–388, http://dx.doi.org/10.1002/jbio.200910021.
- Chen R., Rudney J., Aparicio C., Fok A., Jones R.S. Quantifying dental biofilm growth using cross-polarization optical coherence tomography. Lett Appl Microbiol 2012; 54(6): 537–542, http://dx.doi.org/10.1111/j.1472-765X.2012.03243.x.
- Garcez A.S., Suzuki S.S., Ribeiro M.S., Mada E.Y., Freitas A.Z., Suzuki H. Biofilm retention by 3 methods of ligation on orthodontic brackets: a microbiologic and optical coherence tomography analysis. Am J Orthod Dentofacial Orthop 2011; 140(4): e193–e198, http://dx.doi.org/10.1016/j.ajodo.2011.04.019.
- Mandurah M.M., Sadr A., Bakhsh T.A., Shimada Y., Sumi Y., Tagami J. Characterization of transparent dentin in attrited teeth using optical coherence tomography. Lasers Med Sci 2014, [Epub ahead of print], http://dx.doi.org/10.1007/s10103-014-1541-4.
- Chew H.P., Zakian C.M., Pretty I.A., Ellwood R.P. Measuring initial enamel erosion with quantitative light-induced fluorescence and optical coherence tomography: an in vitro validation study. Caries Res 2014; 48(3): 254–262, http://dx.doi.org/10.1159/000354411.
- Braz A.K., de Araujo R.E., Ohulchanskyy T.Y., Shukla S., Bergey E.J., Gomes A.S.L., Prasad P.N. In situ gold nanoparticles formation: contrast agent for dental optical coherence tomography. J Biomed Opt 2012; 17(6): 066003, http://dx.doi.org/10.1117/1.JBO.17.6.066003.
- Hariri I., Sadr A., Shimada Y., Tagami J., Sumi Y. Effects of structural orientation of enamel and dentine on light attenuation and local refractive index: an optical coherence tomography study. J Dent 2012; 40(5): 387–396, http://dx.doi.org/10.1016/j.jdent.2012.01.017.
- Manesh S.K., Darling C.L., Fried D. Nondestructive assessment of dentin demineralization using polarization-sensitive optical coherence tomography after exposure to fluoride and laser irradiation. J Biomed Mater Res B Appl Biomater 2009; 90B(2): 802–812, http://dx.doi.org/10.1002/jbm.b.31349.
- Manesh S.K., Darling C.L., Fried D. Imaging natural and artificial demineralization on dentin surfaces with polarization sensitive optical coherence tomography. Proc. SPIE, Lasers in Dentistry XIV 2008; 6843: 68430M, http://dx.doi.org/10.1117/12.778788.
- Brady E., Mannocci F., Brown J., Wilson R., Patel S. A comparison of cone beam computed tomography and periapical radiography for the detection of vertical root fractures in nonendodontically treated teeth. Int Endod J 2014; 47(8): 735–746, http://dx.doi.org/10.1111/iej.12209.
- Shemesh H., van Soest G., Wu M.-K., Wesselink P.R. Diagnosis of vertical root fractures with optical coherence tomography. J Endod 2008; 34(6): 739–742, http://dx.doi.org/10.1016/j.joen.2008.03.013.
- Amaechi B.T., Podoleanu A., Higham S.M., Jackson D.A. Correlation of quantitative light-induced fluorescence and optical coherence tomography applied for detection and quantification of early dental caries. J Biomed Opt 2003; 8(4): 642–647, http://dx.doi.org/10.1117/1.1606685.
- Clarkson D.M. An update on optical coherence tomography in dentistry. Dent Update 2014; 41(2): 174–176, 179–180.
- Holtzman J.S., Ballantine J., Fontana M., Wang A., Calantog A., Benavides E., Gonzalez-Cabezas C., Chen Z., Wilder-Smith P. Assessment of early occlusal caries pre- and post-sealant application — an imaging approach. Lasers Surg Med 2014; 46(6): 499–507, http://dx.doi.org/10.1002/lsm.22249.
- Nakajima Y., Shimada Y., Sadr A., Wada I., Miyashin M., Takagi Y., Tagami J., Sumi Y. Detection of occlusal caries in primary teeth using swept source optical coherence tomography. J Biomed Opt 2014 Jan; 19(1): 16020, http://dx.doi.org/10.1117/1.JBO.19.1.016020.
- Chan K.H., Chan A.C., Fried W.A., Simon J.C., Darling C.L., Fried D. Use of 2D images of depth and integrated reflectivity to represent the severity of demineralization in cross-polarization optical coherence tomography. J Biophotonics 2013, [Epub ahead of print], http://dx.doi.org/10.1002/jbio.201300137.
- Gomez J., Zakian C., Salsone S., Pinto S.C.S., Taylor A., Pretty I.A., Ellwood R. In vitro performance of different methods in detecting occlusal caries lesions. J Dent 2013; 41(2): 180–186, http://dx.doi.org/10.1016/j.jdent.2012.11.003.
- Nakagawa H., Sadr A., Shimada Y., Tagami J., Sumi Y. Validation of swept source optical coherence tomography (SS-OCT) for the diagnosis of smooth surface caries in vitro. J Dent 2013; 41(1): 80–89, http://dx.doi.org/10.1016/j.jdent.2012.10.007.
- Nazari A., Sadr A., Campillo-Funollet M., Nakashima S., Shimada Y., Tagami J., Sumi Y. Effect of hydration on assessment of early enamel lesion using swept-source optical coherence tomography. J Biophotonics 2013; 6(2): 171–177, http://dx.doi.org/10.1002/jbio.201200012.
- de Azevedo C.S., Trung L.C.E., Simionato M.R.L., de Freitas A.Z., Matos A.B. Evaluation of caries-affected dentin with optical coherence tomography. Braz Oral Res 2011; 25(5): 407–413, http://dx.doi.org/10.1590/S1806-83242011000500006.
- Chen Y., Otis L., Zhu Q. Polarization memory effect in optical coherence tomography and dental imaging application. J Biomed Opt 2011; 16(8): 086005, http://dx.doi.org/10.1117/1.3606573.
- Kang H., Jiao J.J., Lee C., Le M.H., Darling C.L., Fried D. Nondestructive assessment of early tooth demineralization using cross-polarization optical coherence tomography. IEEE J Sel Top Quantum Electron 2010; 16(4): 870–876, http://dx.doi.org/10.1109/JSTQE.2009.2033610.
- Holtzman J.S., Osann K., Pharar J., Lee K., Ahn Y.C., Tucker T., Sabet S., Chen Z., Gukasyan R., Wilder-Smith P. Ability of optical coherence tomography to detect caries beneath commonly used dental sealants. Lasers Surg Med 2010; 42(8): 752–759, http://dx.doi.org/10.1002/lsm.20963.
- Huminicki A., Dong C., Cleghorn B., Sowa M., Hewko M., Choo-Smith L.P. Determining the effect of calculus, hypocalcification, and stain on using optical coherence tomography and polarized Raman spectroscopy for detecting white spot lesions. Int J Dent 2010; 2010: 879252, http://dx.doi.org/10.1155/2010/879252.
- Shimada Y., Sadr A., Burrow M.F., Tagami J., Ozawa N., Sumi Y. Validation of swept-source optical coherence tomography (SS-OCT) for the diagnosis of occlusal caries. J Dent 2010; 38(8): 655–665, http://dx.doi.org/10.1016/j.jdent.2010.05.004.
- Maia A.M., Fonsêca D.D., Kyotoku B.B., Gomes A.S. Characterization of enamel in primary teeth by optical coherence tomography for assessment of dental caries. Int J Paediatr Dent 2010; 20(2): 158–164, http://dx.doi.org/10.1111/j.1365-263X.2009.01025.x.
- Tao Y.C., Fried D. Selective removal of natural occlusal caries by coupling near-infrared imaging with a CO2 laser. Proc. SPIE, Lasers in Dentistry XIV 2008; 6843: 68430I, http://dx.doi.org/10.1117/12.778790.
- Jones R.S., Darling C.L., Featherstone J.D., Fried D. Imaging artificial caries on the occlusal surfaces with polarization-sensitive optical coherence tomography. Caries Res 2006; 40(2): 81–89, http://dx.doi.org/10.1159/000091052.
- Fried D., Xie J., Shafi S., Featherstone J.D.B., Breunig T.M., Le C. Imaging caries lesions and lesion progression with polarization sensitive optical coherence tomography. J Biomed Opt 2002; 7(4): 618–627, http://dx.doi.org/10.1117/1.1509752.
- Amaechi B.T., Higham S.M., Podoleanu A.G., Rogers J.A., Jackson D.A. Use of optical coherence tomography for assessment of dental caries: quantitative procedure. J Oral Rehabil 2001; 28(12): 1092–1093, http://dx.doi.org/10.1046/j.1365-2842.2001.00840.x.
- Colston B.W.Jr., Sathyam U.S., DaSilva L.B., Everett M.J., Stroeve P., Otis L.L. Dental OCT. Opt Express 1998; 3(6): 230–238, http://dx.doi.org/10.1364/oe.3.000230.
- Darling C.L., Staninec M., Chan K.H., Kang H., Fried D. Remineralization of root caries monitored using cross-polarization optical coherence tomography. Proc. SPIE, Lasers in Dentistry XVIII 2012; 8208: 82080V, http://dx.doi.org/10.1117/12.914633.
- Manesh S.K., Darling C.L., Fried D. Polarization-sensitive optical coherence tomography for the nondestructive assessment of the remineralization of dentin. J Biomed Opt 2009; 14(4): 044002, http://dx.doi.org/10.1117/1.3158995.
- Manesh S.K., Darling C.L., Fried D. Assessment of dentin remineralization with PS-OCT. Proc. SPIE, Lasers in Dentistry XV 2009; 7162: 71620W, http://dx.doi.org/10.1117/12.816865.
- Natsume Y., Nakashima S., Sadr A., Shimada Y., Tagami J., Sumi Y. Estimation of lesion progress in artificial root caries by swept source optical coherence tomography in comparison to transverse microradiography. J Biomed Opt 2011; 16(7): 071408, http://dx.doi.org/10.1117/1.3600448.
- Chong S.L., Darling C.L., Fried D. Nondestructive measurement of the inhibition of demineralization on smooth surfaces using polarization-sensitive optical coherence tomography. Lasers Surg Med 2007; 39(5): 422–427, http://dx.doi.org/10.1002/lsm.20506.
- Chong S.L. Detection of white spot lesions around orthodontic brackets using polarization-sensitive optical coherence tomography. Am J Orthod Dentofacial Orthop 2007; 132(5): 711, http://dx.doi.org/10.1016/j.ajodo.2007.02.032.
- Lee R.C., Kang H., Darling C.L., Fried D. Automated assessment of the remineralization of artificial enamel lesions with polarization-sensitive optical coherence tomography. Biomed Opt Express 2014; 5(9): 2950–2962, http://dx.doi.org/10.1364/BOE.5.002950.
- Mandurah M.M., Sadr A., Shimada Y., Kitasako Y., Nakashima S., Bakhsh T.A., Tagami J., Sumi Y. Monitoring remineralization of enamel subsurface lesions by optical coherence tomography. J Biomed Opt 2013; 18(4): 046006, http://dx.doi.org/10.1117/1.JBO.18.4.046006.
- Kang H., Darling C.L., Fried D. Nondestructive monitoring of the repair of enamel artificial lesions by an acidic remineralization model using polarization-sensitive optical coherence tomography. Dent Mater 2012; 28(5): 488–494, http://dx.doi.org/10.1016/j.dental.2011.11.020.
- Jones R.S., Fried D. Remineralization of enamel caries can decrease optical reflectivity. J Dent Res 2006; 85(9): 804–808, http://dx.doi.org/10.1177/154405910608500905.
- Benson P.E., Shah A.A., Willmot D.R. Polarized versus nonpolarized digital images for the measurement of demineralization surrounding orthodontic brackets. The Angle Orthodontist 2008; 78(2): 288–293, http://dx.doi.org/10.2319/121306-511.1.
- Kotaku M., Murayama R., Shimamura Y., Takahashi F., Suzuki T., Kurokawa H., Miyazaki M. Evaluation of the effects of fluoride-releasing varnish on dentin demineralization using optical coherence tomography. Dent Mater J 2014; 33(5): 648–655, http://dx.doi.org/10.4012/dmj.2014-072.
- Arnaud T.M.S., de Barros Neto B., Diniz F.B. Chitosan effect on dental enamel de-remineralization: an in vitro evaluation. J Dent 2010; 38(11): 848–852, http://dx.doi.org/10.1016/j.jdent.2010.06.004.
- Lee C., Darling C.L., Fried D. Polarization-sensitive optical coherence tomographic imaging of artificial demineralization on exposed surfaces of tooth roots. Dent Mater 2009; 25(6): 721–728, http://dx.doi.org/10.1016/j.dental.2008.11.014.
- Hsu D.J., Darling C.L., Lachica M.M., Fried D. Nondestructive assessment of the inhibition of enamel demineralization by CO2 laser treatment using polarization sensitive optical coherence tomography. J Biomed Opt 2008; 13(5): 054027, http://dx.doi.org/10.1117/1.2976113.
- Can A.M., Darling C.L., Ho C., Fried D. Non-destructive assessment of inhibition of demineralization in dental enamel irradiated by a λ=9.3-microm CO2 laser at ablative irradiation intensities with PS-OCT. Lasers Surg Med 2008; 40(5): 342–349, http://dx.doi.org/10.1002/lsm.20633.
- Hsu D.J., Darling C.L., Fried D. Imaging laser irradiated enamel surfaces with polarization sensitive optical coherence tomography. Proc. SPIE, Lasers in Dentistry XIV 2008; 6843: 8430N, http://dx.doi.org/10.1117/12.778789.
- Pande P., Shrestha S., Park J., Serafino M.J., Gimenez-Conti I., Brandon J., Cheng Y.S., Applegate B.E., Jo J.A. Automated classification of optical coherence tomography images for the diagnosis of oral malignancy in the hamster cheek pouch. J Biomed Opt 2014; 19(8): 086022, http://dx.doi.org/10.1117/1.JBO.19.8.086022.
- Hamdoon Z., Jerjes W., Upile T., McKenzie G., Jay A., Hopper C. Optical coherence tomography in the assessment of suspicious oral lesions: an immediate ex vivo study. Photodiagnosis Photodyn Ther 2013; 10(1): 17–27, http://dx.doi.org/10.1016/j.pdpdt.2012.07.005.
- Adegun O.K., Tomlins P.H., Hagi-Pavli E., Bader D.L., Fortune F. Quantitative optical coherence tomography of fluid-filled oral mucosal lesions. Lasers Med Sci 2013; 28(5): 1249–1255, http://dx.doi.org/10.1007/s10103-012-1208-y.
- Park K.J., Schneider H., Haak R. Assessment of interfacial defects at composite restorations by swept source optical coherence tomography. J Biomed Opt 2013; 18(7): 076018, http://dx.doi.org/10.1117/1.JBO.18.7.076018.
- Nazari A., Sadr A., Shimada Y., Tagami J., Sumi Y. 3D assessment of void and gap formation in flowable resin composites using optical coherence tomography. J Adhes Dent 2013; 15(3): 237–243, http://dx.doi.org/10.3290/j.jad.a28623.
- Shimada Y., Nakagawa H., Sadr A., Wada I., Nakajima M., Nikaido T., Otsuki M., Tagami J., Sumi Y. Noninvasive cross-sectional imaging of proximal caries using swept-source optical coherence tomography (SS-OCT) in vivo. J Biophotonics 2014; 7(7): 506–513, http://dx.doi.org/10.1002/jbio.201200210.
- Lammeier C., Li Y., Lunos S., Fok A., Rudney J., Jones R.S. Influence of dental resin material composition on cross-polarization-optical coherence tomography imaging. J Biomed Opt 2012; 17(10): 106002, http://dx.doi.org/10.1117/1.JBO.17.10.106002.
- Nazari A., Sadr A., Saghiri M.A., Campillo-Funollet M., Hamba H., Shimada Y., Tagami J., Sumi Y. Non-destructive characterization of voids in six flowable composites using swept-source optical coherence tomography. Dent Mater 2013; 29(3): 278–286, http://dx.doi.org/10.1016/j.dental.2012.11.004.
- Bakhsh T.A., Sadr A., Shimada Y., Mandurah M.M., Hariri I., Alsayed E.Z., Tagami J., Sumi Y. Concurrent evaluation of composite internal adaptation and bond strength in a class-I cavity. J Dent 2013; 41(1): 60–70, http://dx.doi.org/10.1016/j.jdent.2012.10.003.
- Shimada Y., Sadr A., Nazari A., Nakagawa H., Otsuki M., Tagami J., Sumi Y. 3D evaluation of composite resin restoration at practical training using swept-source optical coherence tomography (SS-OCT). Dent Mater J 2012; 31(3): 409–417, http://dx.doi.org/10.4012/dmj.2011-244.
- Monteiro G.Q.M., Montes M.A.J.R., Gomes A.S.L., Mota C.C.B.O., Campello S.L., Freitas A.Z. Marginal analysis of resin composite restorative systems using optical coherence tomography. Dent Mater 2011; 27(12): e213–e23, http://dx.doi.org/10.1016/j.dental.2011.08.400.
- Senawongse P., Pongprueksa P., Harnirattisai C., Sumi Y., Otsuki M., Shimada Y., Tagami J. Non-destructive assessment of cavity wall adaptation of class V composite restoration using swept-source optical coherence tomography. Dent Mater J 2011; 30(4): 517–522, http://dx.doi.org/10.4012/dmj.2011-061.
- Bakhsh T.A., Sadr A., Shimada Y., Tagami J., Sumi Y. Non-invasive quantification of resin-dentin interfacial gaps using optical coherence tomography: validation against confocal microscopy. Dent Mater 2011; 27(9): 915–925, http://dx.doi.org/10.1016/j.dental.2011.05.003.
- Ishibashi K., Ozawa N., Tagami J., Sumi Y. Swept-source optical coherence tomography as a new tool to evaluate defects of resin-based composite restorations. J Dent 2011; 39(8): 543–548, http://dx.doi.org/10.1016/j.jdent.2011.05.005.
- Makishi P., Shimada Y., Sadr A., Tagami J., Sumi Y. Non-destructive 3D imaging of composite restorations using optical coherence tomography: marginal adaptation of self-etch adhesives. J Dent 2011; 39(4): 316–325, http://dx.doi.org/10.1016/j.jdent.2011.01.011.
- Matheus T.C., Kauffman C.M., Braz A.K., Mota C.C., Gomes A.S. Fracture process characterization of fiberreinforced dental composites evaluated by optical coherence tomography, SEM and optical microscopy. Braz Dent J 2010; 21(5): 420–427, http://dx.doi.org/10.1590/S0103-64402010000500008.
- de Melo L.S.A., de Araujo R.E., Freitas A.Z., Zezell D., Vieira N.D., Girkin J., Hall A., Carvalho M.T., Gomes A.S.L. Evaluation of enamel dental restoration interface by optical coherence tomography. J Biomed Opt 2005; 10(6): 064027, http://dx.doi.org/10.1117/1.2141617.
- Braz A.K.S., Aguiar C.M., Gomes A.S.L. Evaluation of the integrity of dental sealants by optical coherence tomography. Dent Mater 2011; 27(4): e60–e64, http://dx.doi.org/10.1016/j.dental.2010.11.010.
- Jones R.S., Staninec M., Fried D. Imaging artificial caries under composite sealants and restorations. J Biomed Opt 2004; 9(6): 1297–1304, http://dx.doi.org/10.1117/1.1805562.
- Otis L.L., al-Sadhan R.I., Meiers J., Redford-Badwal D. Identification of occlusal sealants using optical coherence tomography. J Clin Dent 2003; 14(1): 7–10.
- Minamino T., Mine A., Omiya K., Matsumoto M., Nakatani H., Iwashita T., Ohmi M., Awazu K., Yatani H. Nondestructive observation of teeth post core space using optical coherence tomography: a pilot study. J Biomed Opt 2014; 19(4): 046004, http://dx.doi.org/10.1117/1.JBO.19.4.046004.
- Shemesh H., van Soest G., Wu M.-K., van der Sluis L.W.M., Wesselink P.R. The ability of optical coherence tomography to characterize the root canal walls. J Endod 2007; 33(11): 1369–1373, http://dx.doi.org/10.1016/j.joen.2007.06.022.
- Kikuchi K., Akiba N., Sadr A., Sumi Y., Tagami J., Minakuchi S. Evaluation of the marginal fit at implant — abutment interface by optical coherence tomography. J Biomed Opt 2014; 19(5): 055002, http://dx.doi.org/10.1117/1.JBO.19.5.055002.
- Bista B., Sadr A., Nazari A., Shimada Y., Sumi Y., Tagami J. Nondestructive assessment of current one-step self-etch dental adhesives using optical coherence tomography. J Biomed Opt 2013; 18(7): 76020, http://dx.doi.org/10.1117/1.JBO.18.7.076020.
- Drexler W., Fujimoto J.G. Optical coherence tomography. Springer Berlin Heidelberg; 2008, http://dx.doi.org/10.1007/978-3-540-77550-8.
- Xiang X., Sowa M.G., Iacopino A.M., Maev R.G., Hewko M.D., Man A., Liu K.-Z. An update on novel non-invasive approaches for periodontal diagnosis. J Periodontol 2010; 81(2): 186–198, http://dx.doi.org/10.1902/jop.2009.090419.
- Sumi Y., Ozawa N., Nagaosa S., Minakuchi S., Umemura O. Application of optical coherence tomography (OCT) to nondestructive inspection of dentures. Arch Gerontol Geriatr 2011; 53(2): 237–241, http://dx.doi.org/10.1016/j.archger.2010.11.022.
- Lin C.-L., Kuo W.-C., Chang Y.-H., Yu J.-J., Lin Y.-C. Examination of ceramic/enamel interfacial debonding using acoustic emission and optical coherence tomography. Dent Mater 2014; 30(8): 910–916, http://dx.doi.org/10.1016/j.dental.2014.05.023.
- Nakajima Y., Shimada Y., Miyashin M., Takagi Y., Tagami J., Sumi Y. Noninvasive cross-sectional imaging of incomplete crown fractures (cracks) using swept-source optical coherence tomography. Int Endod J 2012; 45(10): 933–941, http://dx.doi.org/10.1111/j.1365-2591.2012.02052.x.
- Turkistani A., Sadr A., Shimada Y., Nikaido T., Sumi Y., Tagami J. Sealing performance of resin cements before and after thermal cycling: evaluation by optical coherence tomography. Dent Mater 2014; 30(9): 993–1004, http://dx.doi.org/10.1016/j.dental.2014.05.010.
- Lin C.-L., Kuo W.-C., Yu J.-J., Huang S.-F. Examination of ceramic restorative material interfacial debonding using acoustic emission and optical coherence tomography. Dent Mater 2013; 29(4): 382–388, http://dx.doi.org/10.1016/j.dental.2012.12.003.
- Na J., Lee B.H., Baek J.H., Choi E.S. Optical approach for monitoring the periodontal ligament changes induced by orthodontic forces around maxillary anterior teeth of white rats. Med Biol Eng Comput 2008; 46(6): 597–603, http://dx.doi.org/10.1007/s11517-007-0300-0.
- Baek J.H., Na J., Lee B.H., Choi E.S., Sone W.S. Optical approach to the periodontal ligament under orthodontic tooth movement: a preliminary study with optical coherence tomography. Am J Orthod Dentofacial Orthop 2009; 135(2): 252–259, http://dx.doi.org/10.1016/j.ajodo.2007.10.037.
- Pithon M.M., dos Santos M.J., Andrade C.S.S., Leão Filho J.C., Braz A.K., de Araujo R.E., Tanaka O.M., Fidalgo T.K., dos Santos A.M., Maia L.C. Effectiveness of varnish with CPP–ACP in prevention of caries lesions around orthodontic brackets: an OCT evaluation. Eur J Orthod 2014, [Epub ahead of print], http://dx.doi.org/10.1093/ejo/cju031.
- Koprowski R., Machoy M., Woźniak K., Wróbel Z. Automatic method of analysis of OCT images in the assessment of the tooth enamel surface after orthodontic treatment with fixed braces. Biomed Eng Online 2014; 13: 48, http://dx.doi.org/10.1186/1475-925X-13-48.
- Isfeld D.M., Aparicio C., Jones R.S. Assessing near infrared optical properties of ceramic orthodontic brackets using cross-polarization optical coherence tomography. J Biomed Mater Res B Appl Biomater 2014; 102(3): 516–523, http://dx.doi.org/10.1002/jbm.b.33029.
- Leão Filho J.C.B., Braz A.K.S., de Souza T.R., de Araujo R.E., Pithon M.M., Tanaka O.M. Optical coherence tomography for debonding evaluation: an in-vitro qualitative study. Am J Orthod Dentofacial Orthop 2013; 143(1): 61–68, http://dx.doi.org/10.1016/j.ajodo.2012.08.025.
- Marcauteanu C., Negrutiu M., Sinescu C., Demjan E., Huges M., Bradu A., Dobre G., Podoleanu A.G. Occlusal overload investigations by noninvasive technology: fluorescence microscopy and en-face optical coherence tomography. Proc. SPIE, Optical Coherence Tomography and Coherence Techniques IV 2009; 7372: 737227, http://dx.doi.org/10.1117/12.831797.
- Mărcăuteanu C., Negrutiu M., Sinescu C., Demjan E., Huges M., Bradu A., Dobre G., Podoleanu A.G. Early detection of tooth wear by en-face optical coherence tomography. Proc. SPIE, Lasers in Dentistry XV 2009; 7162: 716205, http://dx.doi.org/10.1117/12.809828.
- Demjan E., Mărcăuţeanu C., Bratu D., Sinescu C., Negruţiu M., Ionita C., Topală F., Hughes M., Bradu A., Dobre G., Podoleanu A.G. Analysis of dental abfractions by optical coherence tomography. Proc. SPIE, Lasers in Dentistry XVI 2010; 7549: 754903, http://dx.doi.org/10.1117/12.842819.
- The Academy of Prosthodontics. The glossary of prosthodontic terms. 8th ed. St. Louis; 2005.
- Smith B.G., Knight J.K. An index for measuring the wear of teeth. Br Dent J 1984; 156(12): 435–438, http://dx.doi.org/10.1038/sj.bdj.4805394.
- Mărcăuţeanu C., Topală F., Negrutiu M.L., Stoica E.T., Sinescu C. 3D finite element analysis of restorative materials used in dental abfractions. Solid State Phenomena 2012; 188: 82–86, http://dx.doi.org/10.4028/www.scientific.net/ssp.188.82.
- Marcauteanu C., Negrutiu M., Sinescu C., Stoica E.T., Ionita C., Topala F., Vasile L., Bradu A., Dobre G., Podoleanu A.G. Early characterization of occlusal overloaded cervical dental hard tissues by en face optical coherence tomography. Proc. SPIE, Optical Coherence Tomography and Coherence Techniques V 2011; 8091: 80911X, http://dx.doi.org/10.1117/12.889711.
- Marcauteanu C., Bradu A., Sinescu C., Topala F.I., Negrutiu M.L., Podoleanu A.G. Quantitative evaluation of dental abfraction and attrition using a swept-source optical coherence tomography system. J Biomed Opt 2014; 19(2): 021108, http://dx.doi.org/10.1117/1.JBO.19.2.021108.
- Stoica E.T., Marcauteanu C., Bradu A., Sinescu C., Topala F.I., Negrutiu M.L., Duma V.F., Podoleanu A.G. Imaging of noncarious cervical lesions by means of a fast swept source optical coherence tomography system. Proc. SPIE, Fifth International Conference on Lasers in Medicine: Biotechnologies Integrated in Daily Medicine 2014; 8925: 89250Y, http://dx.doi.org/10.1117/12.2044214.
- Marcauteanu C., Bradu A., Sinescu C., Topala F.I., Negrutiu M.L., Duma V.F., Podoleanu A.G. The advantages of a swept source optical coherence tomography system in the evaluation of occlusal disorders. Proc. SPIE, Fifth International Conference on Lasers in Medicine: Biotechnologies Integrated in Daily Medicine 2014; 8925: 89250W, http://dx.doi.org/10.1117/12.2044198.
- Kleinetal T., Wieser W., Eigenwillig C.M., Biedermann B.R., Huber R. Megahertz OCT for ultrawide-field retinal imaging with a 1050 nm Fourier domain mode-locked laser. Opt Express 2011; 19(4): 3044–3062, http://dx.doi.org/10.1364/OE.19.003044.
- Wieser W., Biedermann B.R., Klein T., Eigenwillig C.M., Huber R. Multi-megahertz OCT: high quality 3D imaging at 20 million A-scans and 4.5 GVoxels per second. Opt Express 2010; 18(14): 14685–14704, http://dx.doi.org/10.1364/OE.18.014685.
- Enescu M., Sinescu C., Negrutiu M., Negru R., Marsavina L., Topala F., Rominu R., Petrescu E., Bradu A., Dobre G., Rominu M., Podoleanu A. Amalgam and composite resin interface investigation by optical coherence tomography. Advances in Communications, Computers, Systems, Circuits and Devices 2010; p. 316–322.
- Sinescu C., Marsavina L., Negrutiu M.L., Rusu L.C., Ardelean L., Ionita C., Podoleanu A.G., Rominu M., Topala F.I. Confocal microscopy combined with time domain optical coherence tomography and micro computer tomography in interface evaluation of class II direct composite restoration. Rev Chim 2011; 62(10): 1039–1041.
- Negruţiu M.L., Sinescu C., Topala F., Ionita C., Marcauteanu C., Petrescu E.L., Podoleanu A.G. Imagistic evaluation of direct dental restoration: en face OCT versus SEM and microCT. Proc. SPIE, Optical Coherence Tomography and Coherence Techniques V 2011; 8091: 80911T, http://dx.doi.org/10.1117/12.890021.
- Topala F., Sinescu C., Negrutiu M., Bradu A., Rominu M., Podoleanu A.G. 3D reconstructions of resin dental fillings based on en face OCT images. Advances in Biology, Bioengineering and Environment 2010; p. 19–22.
- Rominu M., Sinescu C., Negrutiu M.L., Rominu R.O., Pop D.M., Topala F., Stoia A., Petrescu E., Bradu A., Dobre G., Podoleanu A.G. Adhesive improvement in optical coherence tomography combined with confocal microscopy for class V cavities investigations. Proc. SPIE, Medical Imaging 2010: Biomedical Applications in Molecular, Structural, and Functional Imaging 2010; 7626: 76260Y, http://dx.doi.org/10.1117/12.844538.
- Marcauteanu C., Negrutiu M.L., Ardelean L., Rusu L.C., Podoleanu A. Evaluation of the prognosis of compomer class V restorations through en face optical coherence tomography. Rev Chim 2012; 63(5): 545–547.
- Sinescu C., Negrutiu M.L., Todea C., Balabuc C., Filip L., Rominu R., Bradu A., Hughes M., Podoleanu A.G. Quality assessment of dental treatments using en-face optical coherence tomography. J Biomed Opt 2008; 13(5): 054065, http://dx.doi.org/10.1117/1.2992593.
- He Y., Wang R.K. Dynamic optical clearing effect of tissue impregnated with hyperosmotic agents and studied with optical coherence tomography. J Biomed Opt 2004; 9(1): 200–206, http://dx.doi.org/10.1117/1.1629682.
- Sinescu C., Marsavina L., Negrutiu M.L., Rusu L.C., Ardelean L., Rominu M., Antoniac I., Topala F.I., Podoleanu A.G. New metallic nanoparticles modified adhesive used for time domain optical coherence tomography evaluation of class II direct composite restoration. Rev Chim 2012; 63(4): 380–383.
- Rominu M., Manescu A., Sinescu C., Negrutiu M.L., Topala F., Rominu R.O., Bradu A., Jackson D.A., Giuliani A., Podoleanu A.G. Zirconia enriched dental adhesive: a solution for OCT contrast enhancement. Demonstrative study by synchrotron radiation microtomography. Dent Mater 2014; 30(4): 417–423, http://dx.doi.org/10.1016/j.dental.2014.01.004.
- Todea C., Balabuc C., Sinescu C., Filip L., Kerezsi C., Calniceanu M., Negrutiu M., Bradu A., Hughes M., Podoleanu A.G. En face optical coherence tomography investigation of apical microleakage after laser-assisted endodontic treatment. Lasers Med Sci 2010; 25(5): 629–639, http://dx.doi.org/10.1007/s10103-009-0680-5.
- Todea C., Podoleanu A.G., Sinescu C., Balabuc C., Filip L., Negrutiu M. OCT investigation of apical microleakage — a preliminary in vitro study. Lasers Surg Med 2008; Suppl 20: 15.
- Negrutiu M.L., Sinescu C., Hughes M., Bradu A., Todea C., Balabuc C.I., Filip L.M., Podoleanu A.G. Root canal filling evaluation using optical coherence tomography. Proc. SPIE, Biophotonics: Photonic Solutions for Better Health Care 2008; 6991: 69911T, http://dx.doi.org/10.1117/12.780901.
- Negrutiu M.L., Nica L., Sinescu C., Topala F., Ionita C., Bradu A., Petrescu E.L., Pop D.M., Rominu M., Podoleanu A.G. SEM and microCT validation for en face OCT imagistic evaluation of endodontically treated human teeth. Proc. SPIE, Medical Imaging 2011: Physics of Medical Imaging 2011; 7961: 79614W, http://dx.doi.org/10.1117/12.878320.
- Negrutiu M.L., Sinescu C., Topala F., Nica L., Ionita C., Marcauteanu C., Goguta L., Bradu A., Dobre G., Rominu M., Podoleanu A.G. Root canal filling evaluation using optical coherence tomography. Proc. SPIE, Biophotonics: Photonic Solutions for Better Health Care II 2010; 7715: 77151T, http://dx.doi.org/10.1117/12.854842.
- Todea C., Balabuc C., Filip L., Calniceanu M., Bradu A., Highes M., Podoleanu A.G. Investigation of Er:YAG laser root canal irradiation using en-face OCT. Proc. SPIE, Optical Coherence Tomography and Coherence Techniques IV 2009; 7372: 7372_1C, http://dx.doi.org/10.1364/ECBO.2009.7372_1C.
- Negrutiu M., Sinescu C., Topala F., Rominu M., Markovic D., Pop D., Hughes M., Bradu A., Dobre G., Podoleanu A.G. En face optical coherence tomography investigation of interface fiber posts/adhesive cement/root canal wall. Proc. SPIE, Optical Coherence Tomography and Coherence Techniques IV 2009; 7372: 7372_1A, http://dx.doi.org/10.1364/ECBO.2009.7372_1A.
- Mărcăuteanu C., Demjan E., Sinescu C., Negrutiu M., Motoc A., Lighezan R., Vasile L., Hughes M., Bradu A., Dobre G., Podoleanu A.G. Preliminary optical coherence tomography investigation of the temporo-mandibular joint disc. Proc. SPIE, Optical Coherence Tomography and Coherence Domain Optical Methods in Biomedicine XIV 2010; 7554: 75542G, http://dx.doi.org/10.1117/12.842783.
- Sinescu C., Negrutiu M.L., Huges M., Bradu A., Todea C., Rominu R., Dodenciu D., Laissue P.L., Podoleanu A.G. Investigation of bracket bonding for orthodontic treatments using en-face optical coherence tomography. Proc. SPIE, Biophotonics: Photonic Solutions for Better Health Care 2008; 6991: 69911M, http://dx.doi.org/10.1117/12.780701.
- Rominu R.O., Popa M., Sinescu C., Negrutiu M.L., Pop D.M., Rusu L.C., Rominu M., Topala F., Podoleanu A.G. The use of spectral domain optical coherence tomography in orthodontics. Materiale Plastice 2012; 49(2): 99–100.
- Rominu R., Sinescu C., Rominu M., Negrutiu M., Petrescu E., Pop D., Podoleanu A.G. The assessment of orthodontic bonding defects: optical coherence tomography followed by three-dimensional reconstruction. Proc. SPIE, Optical Complex Systems: OCS11 2011; 8172: 817214, http://dx.doi.org/10.1117/12.896772.
- Rominu R.O., Sinescu C., Rominu M., Negrutiu M., Laissue P., Mihali S., Cuc L., Hughes M., Bradu A., Podoleanu A. An innovative approach for investigating the ceramic bracket-enamel interface — optical coherence tomography and confocal microscopy. Proc. SPIE, 1st Canterbury Workshop on Optical Coherence Tomography and Adaptive Optics 2008; 7139: 71390O, http://dx.doi.org/10.1117/12.814894.
- Rominu R.O., Sinescu C., Pop D.M., Hughes M., Bradu A., Rominu M., Podoleanu A.G. En-face optical coherence tomography and fluorescence in evaluation of orthodontic interfaces. World Academy of Science, Engineering and Technology 2009; 3: 591–594.
- Demian D., Duma V.-F., Sinescu C., Negrutiu M.L., Cernat R., Topala F.I., Hutiu G., Bradu A., Podoleanu A.G. Design and testing of prototype handheld scanning probes for optical coherence tomography. Proc Inst Mech Eng H 2014; 228(8):743–753, http://dx.doi.org/10.1177/0954411914543963.
- Sinescu C., Negrutiu M., Tatar R., Terteleac A., Negru R., Hluscu M., Culea L., Rominu M., Marsavina L., Hughes M., Bradu A., Dobre G.M., Marcauteanu C., Demjan E., Podoleanu A.G. Investigation of osteoconductive bone substitute by particles analysis, numerical simulation and optical coherence tomography. Proc. SPIE, Lasers In Dentistry XV 2009; 7162: 716207.
- Negruţiu M.L., Sinescu C., Canjau S., Manescu A., Topală F.I., Hoinoiu B., Romоnu M., Mărcăuţeanu C., Duma V., Bradu A., Podoleanu A.G. Bone regeneration assessment by optical coherence tomography and MicroCT synchrotron radiation. Proc. SPIE, Optical Coherence Tomography and Coherence Techniques VI 2013; 8802: 880204, http://dx.doi.org/10.1117/12.2032624.
- Rusu L.C., Negrutiu M.L., Sinescu C., Hoinoiu B., Topala F.I., Duma V.F., Rominu M., Podoleanu A.G. Time domain optical coherence tomography investigation of bone matrix interface in rat femurs. Proc. SPIE, International Symposium on Photoelectronic Detection and Imaging 2013: Fiber Optic Sensors and Optical Coherence Tomography 2013; 8914: 89141H, http://dx.doi.org/10.1117/12.2036345.
- Sinescu C., Huges M., Bradu A., Negrutiu M., Todea C., Antonie S., Laissue P.L., Rominu M., Podoleanu A.G. Implant bone interface investigated with a non-invasive method: optical coherence tomography. Proc. SPIE, Biophotonics: Photonic Solutions for Better Health Care 2008; 6991: 69911L, http://dx.doi.org/10.1117/12.780697.
- Antonie S., Sinescu C., Negrutiu M., Sticlaru C., Negru R., Laissue F.L., Rominu M., Podoleanu A.G. Investigation of implant bone interface with non-invasive methods: numerical simulation, strain gauges and optical coherence tomography. Key Engineering Materials 2008; 399: 193–198, http://dx.doi.org/10.4028/www.scientific.net/KEM.399.193.
- Negrutiu M.L., Sinescu C., Todea C., Podoleanu A.G. Complete denture analyzed by optical coherence tomography. Proc. SPIE, Lasers in Dentistry XIV 2008; 6843: 68430R, http://dx.doi.org/10.1117/12.767106.
- Sinescu C., Negrutiu M., Todea C., Hughes M., Tudorache F., Podoleanu A.G. Fixed partial denture investigated by optical coherence tomography. Proc. SPIE, Coherence Domain Optical Methods and Optical Coherence Tomography in Biomedicine XII 2008; 6847: 684707, http://dx.doi.org/10.1117/12.766315.
- Fabricky M., Todea C., Sinescu C., Negrutiu M.L., Ardelean L., Rusu L.C., Petrescu E.L., Bratu C., Topala F.I., Podoleanu A.G., Bratu E.A. Integral ceramic inlay evaluation by time domain optical coherence tomography. Rev Chim 2012; 63(6): 633–335.
- Sinescu C., Negrutiu M.L., Rominu R.O., Rusu L.C., Topala F.I., Rominu M., Ardelean L., Podoleanu A. Time domain optical coherence tomography evaluation of polymeric fixed partial prostheses. Materiale Plastice 2012; 49(1): 58–61.
- Sinescu C., Negrutiu M., Topala F., Ionita C., Negru R., Fabriky M., Marcauteanu C., Bradu A., Dobre G., Marsavina L., Rominu M., Podoleanu A. Ceramic and polymeric dental onlays evaluated by photo elasticity, optical coherence tomography and micro computed tomography. Proc. SPIE, Optical Complex Systems: OCS11 2011; 8172: 817208, http://dx.doi.org/10.1117/12.896717.
- Negrutiu M.L., Sinescu C., Draganescu G., Rominu R.O., Rominu M., Rusu L.C., Ardelean L., Pop D.M., Petrescu E.L., Podoleanu A.G., Topala F.I. Laser microspectral analysis for validation of en-face OCT imagistic evaluation of microleakage between the metallic framework and veneer materials in fixed partial prostheses. Rev Chim 2011; 62(10): 1185–1188.
- Petrescu E., Sinescu C., Negrutiu M.L., Rominu R., Pop D.M., Rominu M. Non-invasive imagistic investigation of metal-ceramic crowns. Proc. SPIE, Optical Sensors and Biophotonics II 2011; 7990: 79900Y, http://dx.doi.org/10.1117/12.891278.
- Petrescu E., Sinescu C., Negrutiu M.L., Pop D., Rominu R., Enescu M., Rominu M., Bradu A., Dobre G., Podoleanu A.G. OCT and RX validation of metal-ceramic crowns repaired with ceramic material. Proc. SPIE, Optical Complex Systems: OCS11 2011; 8172: 817213, http://dx.doi.org/10.1117/12.896770.
- Negrutiu M.L., Sinescu C., Topala F.I., Ionita C., Goguta L., Marcauteanu C., Rominu M., Podoleanu A.G. Optical investigations of various polymeric materials used in dental technology. Proc. SPIE, Optical Complex Systems: OCS11 2011; 8172: 817216, http://dx.doi.org/10.1117/12.896932.
- Sinescu C., Negrutiu M.L., Ionita C., Topala F., Petrescu E., Rominu R., Pop D.M., Marsavina L., Negru R., Bradu A., Rominu M., Podoleanu A.G. Radiographic, microcomputer tomography, and optical coherence tomography investigations of ceramic interfaces. Proc. SPIE, Optical Sensors and Biophotonics II 2011; 7990: 79900W, http://dx.doi.org/10.1117/12.890272.
- Sinescu C., Negrutiu M.L., Ionita C., Marsavina L., Negru R., Caplescu C., Bradu A., Topala F., Rominu R.O., Petrescu E., Leretter M., Rominu M., Podoleanu A.G. Morphological characterization of dental prostheses interfaces using optical coherence tomography. Proc. SPIE, Medical Imaging 2010: Biomedical Applications in Molecular, Structural, and Functional Imaging 2010; 7626: 76261P, http://dx.doi.org/10.1117/12.844533.
- Rominu M., Sinescu C., Negrutiu M., Birtea N.M., Petrescu E., Rominu R., Hughes M., Bradu A., Dobre G., Podoleanu A.G. A qualitative approach on marginal adaptation of conditioned dental infrastructures using optical coherence tomography. Proceedings of the 1st International Conference on Manufacturing Engineering, Quality and Production Systems (Vol. I). 2009, p. 255–259.
- Sinescu C., Negrutiu M., Birtea N.M., Petrescu E., Rominu R.O., Marcautean C., Demjan E., Cuc L., Hughes M., Bradu A., Dobre G., Rominu M., Podoleanu A.G. Time domain and spectral optical coherence tomography investigations of integral ceramic fixed partial dentures. Proceedings of the 2nd International Conference on Maritime and Naval Science and Engineering. 2009, p. 77–81.
- Sinescu C., Negrutiu M., Hughes M., Bradu A., Todea C., Rominu M., Laissue P.L., Podoleanu A.G. An optical coherence tomography investigation of material defects in ceramic fixed partial dental prostheses. Proc. SPIE, Biophotonics: Photonic Solutions for Better Health Care 2008; 6991: 69910O, http://dx.doi.org/10.1117/12.780694.
- Negrutiu M.L., Sinescu C., Hughes M., Bradu A., Goguta L., Rominu M., Negru R., Podoleanu A.G. Fibres reinforced dentures investigated with en-face optical coherence tomography. Proc. SPIE, Biophotonics: Photonic Solutions for Better Health Care 2008; 6991: 69911U, http://dx.doi.org/10.1117/12.780904.
- Negrutiu M.L., Sinescu C., Hughes M., Bradu A., Rominu M., Todea C., Dobre G., Podoleanu A.G. Optical coherence tomography and confocal microscopy investigations of dental prostheses. Proc. SPIE, 1st Canterbury Workshop on Optical Coherence Tomography and Adaptive Optics 2008; 7139: 71390N, http://dx.doi.org/10.1117/12.816672.
- Sinescu C, Negruţiu M.L., Petrescu E., Rominu M., Marcauteanu C., Rominu R., Hughes M., Bradu A., Dobre G., Podoleanu A.G. Marginal adaptation of ceramic veneers investigated with en face optical coherence tomography. Proc. SPIE, Optical Coherence Tomography and Coherence Techniques IV 2009; 7372: 73722C, http://dx.doi.org/10.1117/12.831830.
- Duma V.-F., Lee K.-S., Meemon P., Rolland J.P. Experimental investigations of the scanning functions of galvanometer-based scanners with applications in OCT. Appl Opt 2011; 50(29): 5735–5749, http://dx.doi.org/10.1364/AO.50.005735.
- Duma V.-F. Optimal scanning function of a galvanometer scanner for an increased duty cycle. Opt Eng 2010; 49(10): 103001, http://dx.doi.org/10.1117/1.3497570.
- Lu C.D., Kraus M.F., Potsaid B., Choi W., Jayaraman V., Cable A.E., Hornegger J., Duker J.S., Fujimoto J.G. Handheld ultrahigh speed swept source optical coherence tomography instrument using a MEMS scanning mirror. Biomed Opt Express 2014; 5(1): 293–311, http://dx.doi.org/10.1364/BOE.5.000293.
- Jung W., Kim J., Jeon M., Chaney E.J, Stewart C.N., Boppart S.A. Handheld optical coherence tomography scanner for primary care diagnostics. IEEE Trans Biomed Eng 2011; 58(3): 741–744, http://dx.doi.org/10.1109/TBME.2010.2096816.
- Cernat R., Tatla T.S., Pang J., Tadrous P.J., Bradu A., Dobre G., Gelikonov G., Gelikonov V., Podoleanu A.G. Dual instrument for in vivo and ex vivo OCT imaging in an ENT department. Biomed Opt Express 2012; 3: 346–3356, http://dx.doi.org/10.1364/BOE.3.003346.