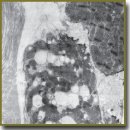
The Effect of Mexidol on Brain Natriuretic Peptide of Cardiomyocytes in a Post-Reperfusion Period in Experiment
Brain natriuretic peptide (BNP) participates in electrolyte balance maintenance in the body playing a critical part in the pathogenesis of cardiovascular diseases, and has the prognostic value in clinical presentation. It is of interest to analyze the peculiarities of BNP interaction with medicinal drugs, e.g. Mexidol, an antihypoxic agent of metabolic type that has a cardioprotective effect and is widely used in cardiology. The effect of Mexidol on BNP in a post-reperfusion period was studied for the first time.
The aim of the investigation was to estimate the effect of Mexidol on BNP accumulation and release intensity in cardiomyocyte granules in rats in a post-reperfusion period.
Materials and Methods. The experiments were carried out on 25 outbred male rats weighing 220–250 g. Total ischemia (10 min) was modeled by cardiovascular bundle compression according to Korpachev. Mexidol was administered intermittently, it being injected intraperitoneally after resuscitation, every 20 min within the first hour. BNP accumulation and release intensity was assessed by a quantitative analysis of immunolabeled granules of atrial myocytes under a transmission electron microscope.
Results. Mexidol administered at a dose of 25 mg/kg body mass within the first hour reperfusion after 10 min of total ischemia has a positive prolonged effect on BNP: after 60 days of a postperfusion period the processes of peptide accumulation and release in atrial myocytes of rats enhance resulting in an additional cardioprotective effect. The increase of BNP release against high synthetic and proliferative activity of fibroblasts contributes to the reduction of cardiosclerosis development in a long-term post-reperfusion period.
The study of immunolabeled granules of BNP myocytes in rat right atrium enabled to discover a new mechanism of a cardioprotective effect of Mexidol in a long-term post-reperfusion period.
Conclusion. Mexidol has a prolonged effect on brain natriuretic peptide and significantly enhances its accumulation and release in atrial cardiomyocytes of rats in a long-term post-reperfusion period having an additional cardioprotective effect and reducing cardiosclerosis development.
- Ogawa T., de Bold A.J. The heart as an endocrine organ. Endocr Connect 2014; 3(2): R31–R44, http://dx.doi.org/10.1530/ec-14-0012.
- Sun Y., Deng T., Lu N., Yan M., Zheng X. B-type natriuretic peptide protects cardiomyocytes at reperfusion via mitochondrial calcium uniporter. Biomed Pharmacother 2010; 64(3): 170–176, http://dx.doi.org/10.1016/j.biopha.2009.09.024.
- Jarai R., Kaun C., Weiss T.W., Speidl W.S., Rychli K., Maurer G., Huber K., Wojta J. Human cardiac fibroblasts express B-type natriuretic peptide: fluvastatin ameliorates its up-regulation by interleukin-1α, tumour necrosis factor-α and transforming growth factor-β. J Cell Mol Med 2009; 13(11–12): 4415–4421, http://dx.doi.org/10.1111/j.1582-4934.2009.00704.x.
- de Bold A.J. Thirty years of research on atrial natriuretic factor: historical background and emerging concepts. Can J Physiol Pharmacol 2011; 89(8): 527–531, http://dx.doi.org/10.1139/y11-019.
- Voulteenaho O., Ala-Kopsala M., Ruskoaho H. BNP as a biomarker in heart disease. Adv Clin Chem 2005; 40: 1–36, http://dx.doi.org/10.1016/s0065-2423(05)40001-3.
- Tang W.H. B-type natriuretic peptide: a critical review. Congest Heart Fail 2007; 13(1): 48–52, http://dx.doi.org/10.1111/j.1527-5299.2007.05622.x.
- Levine Y.C., Rosenberg M.A., Mittleman M., Samuel M., Methachittiphan N., Link M., Josephson M.E., Buxton A.E. B-type natriuretic peptide is a major predictor of ventricular tachyarrhythmias. Heart Rhythm 2014; 11(7): 1109–1116, http://dx.doi.org/10.1016/j.hrthm.2014.04.024.
- Xin W., Lin Z., Mi S. Does B-type natriuretic peptide-guided therapy improve outcomes in patients with chronic heart failure? A systematic review and meta-analysis of randomized controlled trials. Heart Fail Rev 2013; 20(1): 69–80, http://dx.doi.org/10.1007/s10741-014-9437-8.
- Arakawa K., Himeno H., Kirigaya J., Otomo F., Matsushita K., Nakahashi H., Shimizu S., Nitta M., Takamizawa T., Yano H., Endo M., Kanna M., Kimura K., Umemura S. B-type natriuretic peptide as a predictor of ischemia/reperfusion injury immediately after myocardial reperfusion in patients with ST segment elevation acute myocardial infarction. Eur Heart J Acute Cardiovasc Care 2015 Jan [Epub ahead of print], http://dx.doi.org/10.1177/2048872615568964.
- Ejaz N., Khalid M. Utility of brain natriuretic peptide in diagnosis of congestive heart failure and comparison with trans-thoracic echocardiography: a multicenter analysis in South Asian and Arabian population. J Coll Physicians Surg Pak 2015 Jan; 25(1): 12–15.
- Lyu T., Zhao Y., Zhang T., Zhou W., Yang F., Ge H., Ding S., Pu J., He B. Natriuretic peptides as an adjunctive treatment for acute myocardial infarction. Int Heart J 2014 Feb; 55(1): 8–16, http://dx.doi.org/10.1536/ihj.13-109.
- Hu G., Huang X., Zhang K., Jiang H., Hu X. Anti-inflammatory effect of B-type natriuretic peptide postconditioning during myocardial ischemia-reperfusion: involvement of PI3K/Akt signaling pathway. Inflammation 2014; 37(5): 1669–1674, http://dx.doi.org/10.1007/s10753-014-9895-0.
- Shang C. B-type natriuretic peptide-guided therapy for perioperative medicine? Open Heart 2014 Aug; 1(1): e000105, http://dx.doi.org/10.1136/openhrt-2014-000105.
- Toufektzian L., Zisis C., Balaka C., Roussakis A. Effectiveness of brain natriuretic peptide in predicting postoperative atrial fibrillation in patients undergoing non-cardiac thoracic surgery. Interact Cardiovasc Thorac Surg 2015 Jan [Epub ahead of print], http://dx.doi.org/10.1093/icvts/ivu454.
- Blinov D.S., Sernov L.N., Balashov V.P., Blinova E.V., Pivkina L.V., Gogina E.D., Van’kova L.V., Vertyankin M.V., Boyko G.G., Krasilina T.V. Anti-ischemic activity of a new domestic antioxidant — 3-hydroxypyridine ethoxydol derivative. Byulleten’ Eksperimental’noi Biologii i Meditsiny 2011; 152(11): 514–517.
- Zamotaeva M.N., Inchina V.I., Chairkin I.N., Drozdov I.A. Experimental substantiation for the use of mexidol and 3-hydroxypyridine fumarate in chronic myocardial injury. Bull Exp Biol Med 2013; 155(2): 212–213, http://dx.doi.org/10.1007/s10517-013-2115-3.
- Andreeva N.N. The experimental and clinical aspects of the application of Meksidol in hypoxia. Meditsinskiy al’manakh 2009; 4: 193–197.
- Bugrova M.L., Kharkovskaya E.E., Yakovleva E.I. The effect of mexidol on atrial natriuretic peptide in langendorf rat heart preparation. Sovremennye tehnologii v medicine 2014; 6(2): 25–31.
- Bugrova M.L., Yakovleva E.I., Ermolin I.L. The effect of mexidol on synthesis and secretion of atrial natriuretic peptide in cardiac myocytes in early postreperfusion period in experiment. Morfologicheskie vedomosti 2014; 2: 19–25.
- Mukhina I.V., Rakhcheeva M.V., Bugrova M.L. Natriuretic peptides in cardiovascular system regulation. Nizhegorodskiy meditsinskiy zhurnal 2006; 5: 96–102.
- Korpachev V.G., Lysenkov S.P., Tell’ L.Z. Modeling of clinical death and post-resuscitation disease in rats. Patologicheskaya fiziologiya i eksperimental’naya terapiya 1982; 3: 78–80.
- Biserova N.M. Metody vizualizatsii biologicheskikh ul’trastruktur. Podgotovka biologicheskikh ob”ektov dlya izucheniya s pomoshch’yu elektronnykh i fluorestsentnykh konfokal’nykh lazernykh mikroskopov [Imaging methods of biological ultrastructures. Preparation of biological objects for the study using electron and fluorescent confocal laser microscopes]. Moscow: Tovarishchestvo nauchnykh izdaniy KMK; 2013; 104 p.
- Rakhcheeva M.V., Bugrova M.L. Changes in the proportion of A and B-types of granules containing atrial and brain natriuretic peptides in atrial myocytes in vasorenal hypertension in rats. Tsitologiya 2010; 8: 629–633.
- Weidemann A., Klanke B., Wagner M., Volk T., Willam C., Wiesener M., Eckardt K., Warnecke C. Hypoxia, via stabilization of hypoxia-inducible factor HIF-1α is a direct and sufficient stimulus for brain-type natriuretic peptide induction. Biochem J 2008; 409(1): 233–242, http://dx.doi.org/10.1042/bj20070629.
- Arjamaa O., Nikinmaa M. Hypoxia regulates the natriuretic peptide system. Int J Physiol Pathophysiol Pharmacol 2011; 3(3): 191–201.
- Ramos L.W.F., Murad N., Goto E., Antônio E.L., Silva J.A.Jr., Tucci P.F., Carvalho A.C. Ischemia/reperfusion is an independent trigger for increasing myocardial content of mRNA B-type natriuretic peptide. Heart Vessels 2009; 24(6): 454–459, http://dx.doi.org/10.1007/s00380-009-1148-z.
- Bugrova М.L., Yakovleva Е.I., Abrosimov D.А. The relationship of synthesis intensity, accumulation and secretion of natriuretic peptide of atrial myocytes with cardiac rhythm regulation in rats in early postperfusion period. Sovremennye tehnologii v medicine 2012; 3: 26–30.
- Bugrova M.L., Abrosimov D.А., Yakovleva E.I., Baskina О.S., Ermolin I.L. The study on atrial natriuretic peptide of cardiomyocytes in a remote postperfusion period in experiment. Sovremennye tehnologii v medicine 2013; 5(4): 39–44.
- Watson C., Phelan D., Xu M., Collier P., Neary R., Smolenski A., Ledwidge M., McDonald K., Baugh J. Mechanical stretch up-regulates the B-type natriuretic peptide system in human cardiac fibroblasts: a possible defense against transforming growth factor-β mediated fibrosis. Fibrogenesis Tissue Repair 2012; 5(1): 9, http://dx.doi.org/10.1186/1755-1536-5-9.
- Huntley B., Ichiki T., Sangaralingham J., Chen H., Burnett J. B-type natriuretic peptide and extracellular matrix protein interactions in human cardiac fibroblasts. J Cell Physiol 2010 Oct; 225(1), http://dx.doi.org/10.1002/jcp.22253.










