Vasoprotective Effects of Genetically Engineered Biologic Drugs in Patients with Rheumatoid Arthritis
The aim of the investigation was to evaluate the impact of genetically engineered biologic drugs (GEBD) — infliximab and rituximab — on endothelium functional state in patients with rheumatoid arthritis (RA) without any concomitant cardiovascular diseases.
Materials and Мethods. The study involved 77 patients with RA aged from 18 to 50. The patients matched ACR (1987) or ACR/EULAR (2010) classification criteria, had no concomitant cardiovascular diseases, and had at least a two-year RA history. Based on the immunological subtype and the type of therapeutic intervention, the patients were divided into 4 groups. We assessed vasomotor endothelial function at micro- and macrocirculatory levels using AngioScan-01 device (AngioScan-Electronics, Russia) before treatment and after 12 months of treatment.
Results. RA patients were found to have the signs of endothelial dysfunction in micro- and macrocirculatory vasculature, being more prominent in RF/CCPA-positive (rheumatoid factor/cyclic citrullinated peptide antibodies) disease subtype. Endothelial dysfunction manifestations included the decrease of occlusion index in amplitude and the value of phase shift between the channels after a reactive hyperemia test; the values of these parameters correlating to RA duration, DAS 28 (disease activity score), rheumatoid factor level and CCPA concentration. The use of genetically engineered biologic drugs in RA patients was accompanied by a significant decrease of DAS 28 index, as well as by the reduction of endothelial vasomotor dysfunction signs. Both groups of RF/CCPA-negative patients, regardless of a therapeutic intervention type, were found to have the increase of occlusion index in amplitude up to the control values; as compared with the baseline values, the phase shift between the channels increased on average by 1.5 times (p=0.008) during infliximab use, and during rituximab treatment it grew by 1.6 times (p=0.024). In RF/CCPA-positive RA occlusion index in amplitude increased by 23.5% (p=0.005) after infliximab therapy and by 48.8% (p=0.001) during rituximab treatment; the value of phase shift between the channels increased on average by 1.3 times (p=0.028) in RA patients receiving infliximab, while in a group of patients taking rituximab this value was no different from that in the control group.
Conclusion. Genetically engineered biologic drug therapy, along with its high anti-inflammatory activity, produces a vasoprotective effect characterized by improved endothelial functional state in the system of small resistive vessels as well as in large muscular arteries. The higher activity of rituximab endothelial protective effect is achieved in RF/CCPA-positive disease subtype, and the use of infliximab has the greater effect on endothelial function in RF/CCPA-negative RA.
Currently, an important aspect of rheumatoid arthritis (RA) therapy is the problem of cardiovascular pathology due to early development and rapid progression of an arterial sclerotic disease, which is the most common comorbidity in RA patients [1, 2]. The research findings show the manifestations of clinical atherosclerosis to be found in about 20–25% RA patients [3, 4], while subclinical manifestations are revealed in 35–50% patients [5–7], it enables to consider cardiovascular diseases as extraarticular RA manifestations. Thus, atherosclerosis serves as a predictor of unfavorable prognosis, and determines high risk of early death [8].
The mechanisms of accelerated development of atherosclerosis in RA have not been determined yet. Activity of autoimmune inflammation in RA is being discussed as one of possible causes, and considered as the earliest, and therefore, potentially reversible mechanism of endothelial dysfunction, a factor initiating atherogenic processes preceding the formation of macroscopic morphological alteration in arterial walls. Therefore, it is of primary importance to identify the predictors of endothelial dysfunction development and progression at micro- and macrocirculation levels that determines the necessity for developing a common concept of cardiovascular morbidity control and prevention taking into consideration individual RA characteristics. In this view, the priority area of current research is the study of vasoprotective effects of baseline anti-inflammatory therapy including recently brought into actual clinical practice a group of monoclonal antibodies and recombinant proteins, the so called genetically engineered biologic drugs (GEBD), primarily, infliximab and rituximab shown as highly effective drugs to reduce the disease activity and radiographic signs of progressive joint disease in RA [9].
The aim of the investigation was to evaluate the impact of genetically engineered biologic drugs — infliximab and rituximab — on endothelium functional state in patients with rheumatoid arthritis without any concomitant cardiovascular diseases.
Materials and Methods. The study was carried out in accordance with the declaration of Helsinki (adopted in June, 1964 (Helsinki, Finland) and revised in October, 2000 (Edinburg, Scotland)) and approved by the Ethics Committee of Kursk State Medical University. All patients gave their written informed consent.
The study involved 77 patients with a definite RA diagnosis in accordance with ACR (1987) and/or ACR/EULAR (2010) classification criteria.
Inclusion criteria were the following: the age from 18 to 50 years; not exceeding two-year past time duration of RA; active RA for the last 3 months; DAS 28 (disease activity score of RA) as of the time of study involvement being 3.2 scores and higher; preserved capability to self care; the use of any basic anti-inflammatory drugs for the last 3 months before the study initiation; the administration of methotrexat at a stable dose (15–20 mg a week) for minimum 8 weeks before a screening period; the lack of an effect (3 months later) and/or the impossibility to achieve remission (6 months later) against the background of baseline anti-inflammatory therapy at optimal doses.
Exclusion criteria were as follows: low RA activity (DAS 28 being less than 3.2 scores); comorbid cardiovascular pathology (arterial hypertension, any forms of coronary heart disease, chronic heart failure), diabetes mellitus, renal (creatinine level being more than 133 μmol/L) and hepatic diseases (three times and more exceeding levels of AST, ALT, bilirubin), obesity (body mass index being over 30 kg/m2); glucocorticosteroid (GCS) intraarticular injections less than 4 weeks before randomization; intake of prednisolone or its equivalents at the dose of over 20 mg a day; any malignancies including those in past medical history.
Females prevailed among the patients (n=54). 65% patients (n=50) had extraarticular RA manifestations, the most common among them were: rheumatoid nodules (n=38), amyotrophic syndrome (n=34), anemia (n=23), peripheral neuropathy (n=6) and capillarites (n=7). Most RA patients (n=63) before being involved in the study had been given methotrexat (15.0–20.0 mg a week) as baseline anti-inflammatory therapy, 42 patients were administered systemic GCS. Family history of 15 RA patients involved in the study was burdened by cardiovascular diseases, they had moderate (n=46) or low (n=16) cardiovascular risk according to SCORE.
Based on an immunological subtype and the type of therapeutic intervention all RA patients were divided into 4 groups: group 1 consisting of RF/CCPA-negative (rheumatoid factor/cyclic citrullinated peptide antibodies) patients given infliximab (n=20); group 2 including RF/CCPA-negative patients administered rituximab (n=17); RF/CCPA-positive patients given infliximab (n=18) comprised group 3; and group 4 consisted of RF/CCPA-positive patients taking rituximab (n=22). The groups are characterized in the Table.
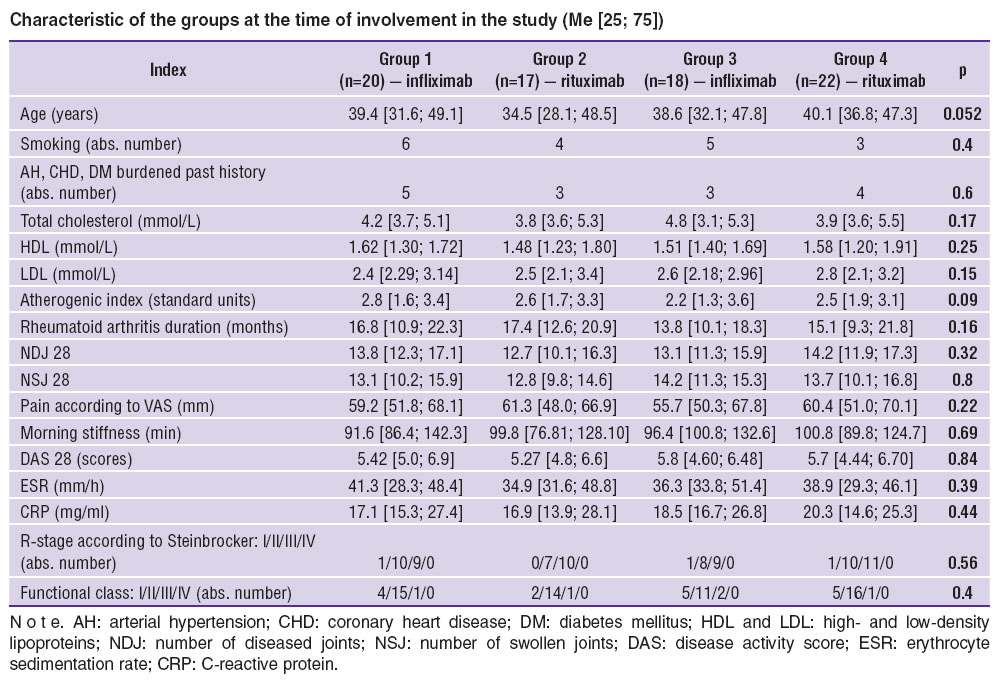 Characteristic of the groups at the time of involvement in the study (Me [25; 75]) Characteristic of the groups at the time of involvement in the study (Me [25; 75])
|
Infliximab was administered at a dose of 3 mg/kg according to the following recommended treatment regimen: 0, after 2, 4, 6 weeks; followed by the intake of every 8 weeks in the course of 12 months. Rituximab was given according to the recommended regimen: 1,000 mg intravenously by drop infusion (within 5–6 h) when involved in the study, 2 weeks later, and then 6 months later the course was repeated (total 4 infusions). All patients under study before and after GEBD therapy were administered a stable dose of methotrexat (12.5–15.0 mg a week). If a patient when involved in the study was administered GCS at a dose calculated as prednisolone not exceeding 15 mg a day, GCS intake was continued.
The intervention efficiency analysis was based on intermediate “surrogate” criteria. To assess clinical efficiency of the therapy we used EULAR criteria based on DAS 28 index changes [1].
A control group involved 46 healthy subjects aged 38.6 [32.1; 47.8] years (among them there were 39 females (84.7%) and 7 males (15.3%)).
Initially and after 12-month therapy all RA patients were examined in accordance with Russian Rheumatology Association recommendations [1].
Endothelial function study included reactive hyperemia test on AngioScan-01 device (AngioScan-Electronics, Russia) according to the recommended requirements for the test execution [10]. Based on the test findings we twice assessed the endothelial function in small resistive (occlusion index in amplitude) and large muscular (phase shift between the channels) arteries: initially (before therapy) and after 12-months GEBD therapy.
Digital data were statistically processed using a software package Microsoft Еxcel and Statistica 6.0. Distribution pattern of variants was determined by Kolmogorv–Smirnov criterion, total variance equation was controlled by F-test. The results were represented as a median (Me) with an interquartile range (25Q; 75Q). To determine the significance of differences between the groups we used nonparametric dispersion analysis (ANOVA) by Kruska–Wallis test (for independent groups) Wilcoxon criterion (for dependent groups). The correlation between the parameters under study was analyzed using Spearman rank correlation analysis (r). In all statistical analysis procedures p=0.05 was taken for a critical significance level of null statistical hypothesis.
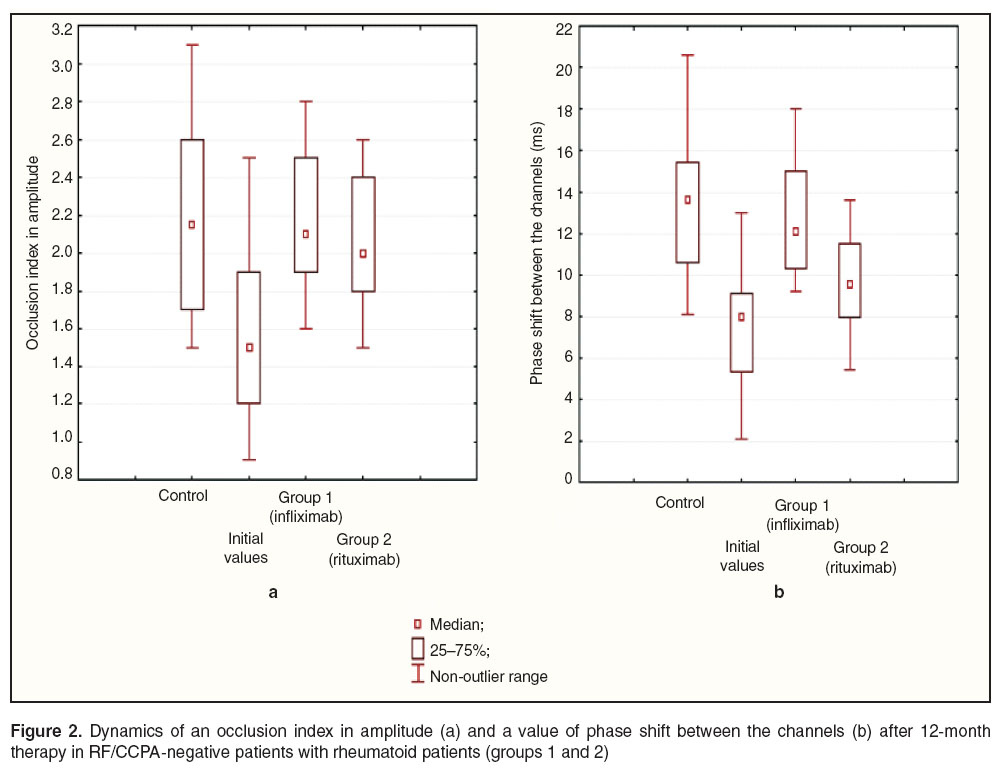
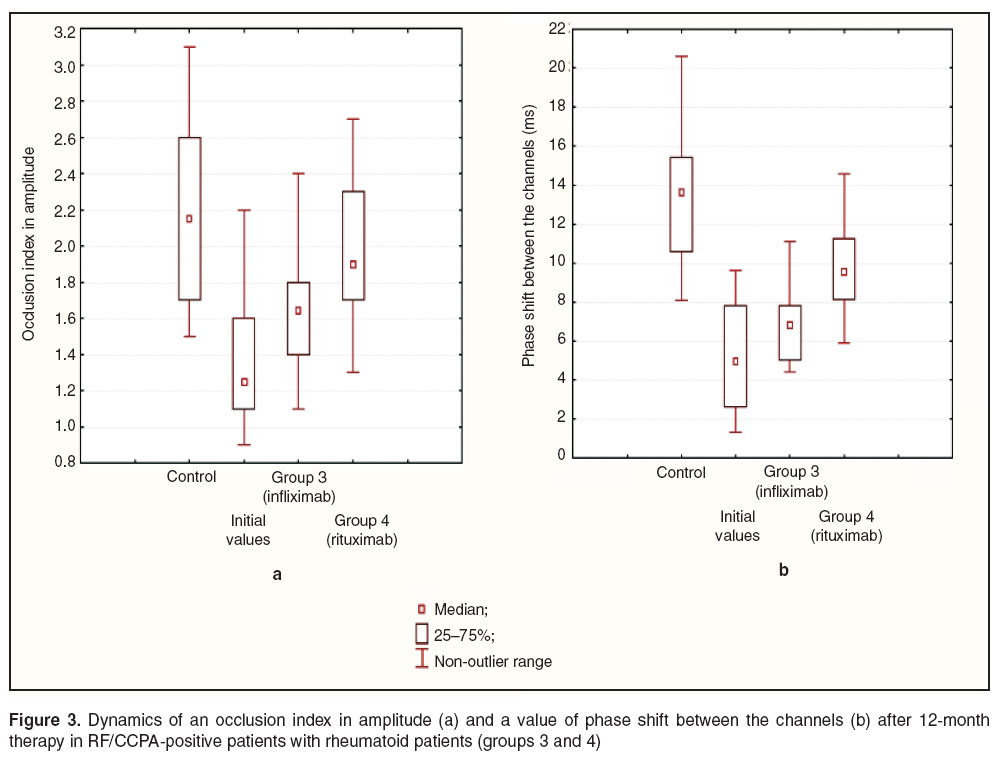
Results and Discussion. The study revealed the signs of micro- and macrocirculatory endothelial dysfunction in RA patients. RF/CCPA-positive patients were found to have initially more marked changes of vasomotor endothelial function, their occlusion index in amplitude and the value of phase shift between the channels being lower than those of controls on average by 1.8 (p=0.024) and by 2.8 times (p=0.001), respectively. RF/CCPA-negative patients appeared to have the decrease of an occlusion index in amplitude on average by 34.5% (p=0.004) and the reduction of a phase shift value between the channels on average by 42.6% (p=0.01) compared to the control.
The correlation analysis revealed the correlation between the value of occlusion index in amplitude, the phase shift in channels and RA duration (r=–0.53, p=0.01; r=–0.42, p=0.022, respectively) and DAS 28 index value (r=–0.68, p=0.01; r=–0.39, p=0.002). There were found the correlations between serum concentration of RF, CCPA IgM and 1) an occlusion index in amplitude (r=–0.52, p=0.01; r=–0.48, p=0.003), as well as 2) the value of phase shift between the channels (r=–0.58, p=0.001; r=–0.54, p=0.02). The findings indicate the contribution of disease-associated factors (activity and duration of chronic immune inflammatory process, RF, CCPA IgM seropositivity) in the mechanisms of endothelial dysfunction formation and progression in RA.
Noninvasive assessment of endothelial function at micro- and macrocirculatory level was performed in accordance with current research trends in a cohort of young and middle-aged RA patients without clinical manifestations of cardiovascular diseases [11, 12]. The resulting data are in agreement with the findings of most previously reported studies: they showed the presence of vasoregulating endothelial dysfunction in RA patients [5–7, 13] including that in the onset of the disease [14], as well as in young patients with a low activity level of an immune inflammatory process [15].
Comparative analysis of GEBD clinical efficiency and their effect on detected vasomotor endothelial dysfunction in RA patients showed the following results: all RA patients had decreased DAS 28 index starting with the 6th week of therapy; by the end of the follow-up 76% patients taking infliximab and 77% patients administered rituximab reached clinical improvement (a good/moderate effect according to EULAR criteria) (Figure 1). In addition, infliximab therapy showed higher efficiency in RF/CCPA-negative patients (group 1), while rituximab administration was accompanied by higher clinical efficiency in RF/CCPA-positive patients (group 4) that is in agreement with the findings of other authors [9, 16, 17].
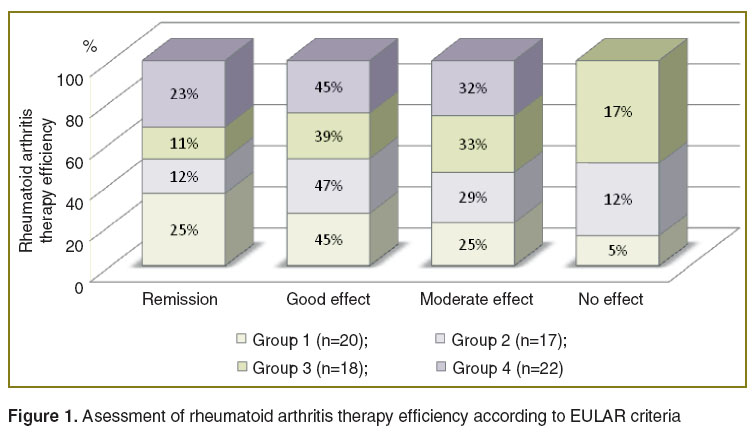 Figure 1. Asessment of rheumatoid arthritis therapy efficiency according to EULAR criteria Figure 1. Asessment of rheumatoid arthritis therapy efficiency according to EULAR criteria
|
The analysis of changes in an occlusion index in amplitude in RF/CCPA-negative patients against the background of a 12-month-therapy showed the index to increase up to its values in the controls in both groups (groups 1 and 2) regardless of the therapy administered (Figure 2 (а)). The findings indicate the recovered endothelial function in microcirculation of patients that can be due to the resolution of disease-mediated inflammation of a vessel wall. In addition, this group of RA patients after infliximab and rituximab therapy were found to have an increased value of phase shift between the channels compared to initial values on average by 1.5 (p=0.008) and 1.6 times (p=0.024), respectively (Figure 2 (b)).
RF/CCPA-positive patients were also found to have an improved functional state of vascular endothelium manifested by the increase of an occlusion index in amplitude by 23.5% (p=0.005) after infliximab therapy, and by 48.8% (p=0.001) against the background of rituximab administration (Figure 3 (а)). Moreover, RA patients given infliximab appeared to have an increase of phase shift between the channels on average by 1.3 times (p=0.028), however, the value was lower by 18.2% (p=0.03) compared to the control (Figure 3 (b)). After rituximab therapy the value of phase shift between the channels reached the control value, the initial one being lower compared to that in RF/CCPA-negative patients.
The results obtained indicate that alongside with high anti-inflammatory activity the administration of infliximab and rituximab in RA patients without cardiovascular pathology is accompanied by a reduced number of vasomotor endothelial dysfunction signs in the system of small resistive vessels and large muscular arteries. Moreover, the efficiency of these drugs differs significantly in RA patients depending on a clinico-immunologic type of the disease. Rituximab showed a higher activity of an anti-inflammatory and vasoprotective effect in RF/CCPA-positive RA; while infliximab had the more significant corrective effect on activity indices of immune inflammation and endothelial dysfunction in RF/CCPA-negative patients.
It should be noted that there are few researches carried out on the problem so far, and their results are rather contradictory. A number of studies [18–20] concerned with the assessment of anti-TNF-α effect on endothelial function in RA showed that infliximab is able to improve endothelium-dependent vasodilatation and have no effect on endothelium-independent vasodilatation; moreover, a positive effect of the drug on endothelial dysfunction appears to be short-term. At the same time, according to some researches carried out in Research Institute of Rheumatology, Russian Academy of Medical Sciences, the decrease of the disease activity results in no change of flow-dependent vasodilatation in this category of patients [9]. The findings on the effect of anti-B-cell therapy on vasoregulating endothelial function are few and preliminary. Rituximab therapy has been shown to be associated with the increase of flow-dependent vasodilatation in RA patients against the background of a decreased level of CRP, DAS 28, the reduction of triglycerides and growth of high-density lipoprotein cholesterol [21–23].
The current contradictions in the assessment of GEBD vasoprotective effects can be due to a number of confounding factors including: errors in selecting patients (insufficient number of follow-ups, lack of randomization, etc.), insufficient duration of a follow-up period, and the most important thing are involved in the study patients with a wide range of concomitant cardiovascular diseases. One of the causes explaining the current situation is that for the first years of GEBD use in clinical practice, their clinical efficiency was primarily studied, while their other properties remained unstudied. All the above determines further studies, which will enable to estimate the GEBD therapy effect on endothelial function in RA.
Conclusion. Revealed signs of endothelial dysfunction in patients with rheumatoid arthritis without concomitant cardiovascular diseases prove the feasibility of implementation of noninvasive endothelial functional state assessment for early diagnosis, determining high cardiovascular risk patient groups, and as one of the therapy efficiency factor. Our results show a long-term therapy of genetically engineered biologic drugs has a corrective effect on endothelial functional state at micro- and macro-vascular levels (in the system of small resistive vessels and in large muscular arteries). High activity of rituximab endothelio-protective effect is achieved in RF/CCPA-positive subtype disease, while infliximab therapy is more effective for endothelial function in RF/CCPA-negative RA. The study findings increase the knowledge on a therapeutic spectrum of the drugs that opens the possibilities for cardiovascular risk control and improve a prognosis in rheumatoid arthritis.
Study Funding. The study was carried out in accordance with a research plan of Kursk State Medical University (state registration number 01201279989).
Conflicts of Interest. The authors have no conflicts of interest related to the present study.
References
- Revmatologiya: klinicheskie rekomendatsii [Rheumatology: clinical recommendations]. Pod red. Nasonova E.L. [Nasonov E.L. (editor)]. Moscow: GEOTAR-Media; 2010; 752 p.
- Kremers H.M., Crowson C.S., Therneau T.M., Roger V.L., Gabriel S.E. High ten-year risk of cardiovascular disease in newly diagnosed rheumatoid arthritis patients: a population-based cohort study. Arthr Rheum 2008; 58(8): 2268–2274, http://dx.doi.org/10.1002/art.23650.
- Naranjo A., Sokka T., Descalzo M.A., Calvo-Alén J., Hørslev-Petersen K., Luukkainen R.K., Combe B., Burmester G.R., Devlin J., Ferraccioli G., Morelli A., Hoekstra M., Majdan M., Sadkiewicz S., Belmonte M., Holmqvist A.C., Choy E., Tunc R., Dimic A., Bergman M., Toloza S., Pincus T.; QUEST-RA Group. Cardiovascular disease in patients with rheumatoid arthritis: results from the QUEST-RA study. Arthritis Res Ther 2008; 10(2): R30, http://dx.doi.org/10.1186/ar2383.
- Wolfe F., Michaud K. The risk of myocardial infarction and pharmacologic and nonpharmacologic myocardial infarction predictors in rheumatoid arthritis: a cohort and nested case-control analysis. Arthr Rheum 2008; 58(9): 2612–2621, http://dx.doi.org/10.1002/art.23811.
- Khan F., Galarraga B., Belch J.J. The role of endothelial function and its assessment in rheumatoid arthritis. Nat Rev Rheumatol 2010; 6(5): 253–261, http://dx.doi.org/10.1038/nrrheum.2010.44.
- Sandoo A., Hodson J., Douglas K.M., Smith J.P., Kitas G.D. The association between functional and morphological assessments of endothelial function in patients with rheumatoid arthritis: a cross-sectional study. Arthritis Res Ther 2013; 15(5): R107, http://dx.doi.org/10.1186/ar4287.
- Knyazeva L.A., Meshcherina N.S., Goryainov I.I., Knyazeva L.I., Stepchenko M.A., Bezgin A.V., Grishina O.V., Ponkratov V.I. Evaluation of arterial wall endothelial function and stiffness in patients with rheumatoid arthritis. Kurskiy nauchno-prakticheskiy vestnik “Chelovek i ego zdorov’e” 2013; 4: 78–84.
- Fietta P., Delsante G. Atherogenesis in rheumatoid arthritis: the “rheumatoid vasculopathy”? Acta Biomed 2009; 80(3): 177–186.
- Genno-inzhenernye biologicheskie preparaty v lechenii revmatoidnogo artrita [Genetically engineered biologic drugs in rheumatoid arthritis therapy]. Pod red. Nasonova E.L. [Nasonov E.L. (editor)]. Moscow: IMA-PRESS; 2013; 552 p.
- Parfenov A.S. Instant diagnosis of cardiovascular diseases. Mir izmereniy 2008; 6: 74–82.
- Kanishcheva E.M., Fedorovich A.A. State estimation possibilities of microvasculature and walls of great vessels. Serdtse 2010; 9(1): 65–70.
- Sandoo A., Veldhuijzen van Zanten J.J., Metsios G.S., Carroll D., Kitas G.D. Vascular function and morphology in rheumatoid arthritis: a systematic review. Rheumatology (Oxford) 2011; 50(11): 2125–2139, http://dx.doi.org/10.1093/rheumatology/ker275.
- Gonzalez-Gay M.A., Gonzalez-Juanatey C. Inflammation, endothelial function and atherosclerosis in rheumatoid arthritis. Arthritis Res Ther 2012; 14: 122, http://dx.doi.org/10.1186/ar3891.
- Bergholm R., Leirisalo-Repo M., Vehkavaara S., Mäkimattila S., Taskinen M.R., Yki-Järvinen H. Impaired responsiveness to NO in newly diagnosed patients with rheumatoid arthritis. Arterioscler Thromb Vasc Biol 2002; 22: 1637–1641, http://dx.doi.org/10.1161/01.ATV.0000033516.73864.4E.
- Vaudo G., Marchesi S., Gerli R., Allegrucci R., Giordano A., Siepi D., Pirro M., Shoenfeld Y., Schillaci G., Mannarino E. Endothelial dysfunction in young patients with rheumatoid arthritis and low disease activity. Ann Rheum Dis 2004; 63(1): 31–35, http://dx.doi.org/10.1136/ard.2003.007740.
- Lukina G.V., Sigidin Ya.A., Pozdnyakova E.S., Aleksandrova E.N., Novikov A.A., Smirnov A.V., Glukhova S.I., Nasonov E.L. Infliximab in Russian clinical practice. Sovremennaya revmatologiya 2012; 3: 37–43.
- Amirdzhanova V.N., Goryachev D.V., Lukina G.V., Kuzikyants K.Kh., Nasonov E.L. The Russian registry of rituximab. Analysis of the efficiency of therapy and the functional state of patients with rheumatoid arthritis. Nauchno-prakticheskaya revmatologiya 48(4): 31–40, http://dx.doi.org/10.14412/1995-4484-2010-1163.
- Komai N., Morita Y., Sakuta T., Kuwabara A., Kashihara N. Anti-tumor necrosis factor therapy increases serum adiponectin levels with the improvement of endothelial dysfunction in patients with rheumatoid arthritis. Mod Rheumatol 2007; 17(5): 385–390, http://dx.doi.org/10.1007/s10165-007-0605-8.
- Gonzalez-Gay M.A., Gonzalez-Juanatey C., Vazquez-Rodriguez T.R., Miranda-Filloy J.A., Llorca J. Insulin resistance in rheumatoid arthritis: the impact of the anti-TNF-alpha therapy. Ann NY Acad Sci 2010; 1193(1): 153–159, http://dx.doi.org/10.1111/j.1749-6632.2009.05287.x.
- Bosello S., Santoliquido A., Zoli A., Di Campli C., Flore R., Tondi P., Ferraccioli G. TNF-alpha blockade induces a reversible but transient effect on endothelial dysfunction in patients with long-standing severe rheumatoid arthritis. Clin Rheumatol 2008; 27(7): 833–839, http://dx.doi.org/10.1007/s10067-007-0803-y.
- Volkov A.V., Lineva O.G., Kuzikyants K.Kh., Lukina G.V., Nasonov E.L. Impact of rituximab therapy on endothelial function and other markers of atherosclerosisin patients with active rheumatoid arthritis. Nauchno-prakticheskaya revmatologiya 2010; 6: 31–36, http://dx.doi.org/10.14412/1995-4484-2010-820.
- Gonzalez-Juanatey C., Llorca J., Vazquez-Rodriguez T.R., Diaz-Varela N., Garcia-Quiroga H., Gonzalez-Gay M.A. Short-term improvement of endothelial function in rituximab-treated rheumatoid arthritis patients refractory to tumor necrosis factor alpha blocker therapy. Arthr Rheum 2008; 59(12): 1821–1824, http://dx.doi.org/10.1002/art.24308.
- Kerekes G., Soltész P., Dér H., Veres K., Szabу Z., Végvári A., Szegedi G., Shoenfeld Y., Szekanecz Z. Effects of rituximab treatment on endothelial dysfunction, carotid atherosclerosis, and lipid profile in rheumatoid arthritis. Clin Rheumatol 2009; 28(6): 705–710, http://dx.doi.org/10.1007/s10067-009-1095-1.