Principles of Manufacturing Biocompatible and Biostable Polymer Implants (Review)
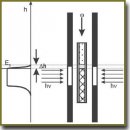
The review concerns the basic theoretical aspects of manufacturing biocompatible and biostable implants and represents, mainly, the experience of our research team. Biocompatible implants have been shown to be understood to mean both: those, which are not rejected by the body as well as those, which are not capsulated in the body. They are to be fabricated according to one-stage frontal photopolymerization with shallow reaction front to avoid defect formation in a polymer. Moreover, an additional operation/stage is required to result in the death of end free macroradicals and labile products in a polymer. For implant fabrication we used photopolimerizable compositions resulting in the formation of hydrophobic spatially cross-linked polymers, their correlation time of rotational motion of a paramagnetic probe of 2,2,6,6-tetramethyl-4-oxypiperidine-1-oxide approximately being 6·10–10 s. The fulfillment of these conditions means the use of radically polymerizable oligomer-based compositions (oligoester methacrylates, oligocarbonate methacrylates, oligourethane methacrylates, etc.). Compositions having lower or higher correlation time of the specified probe are not appropriate for the fabrication of biocompatible and biostable implants. The characteristics of oligomer-monomer compounds have a greater effect on physicochemical properties of implants rather than on their biocompatibility and biostability. No implant incapsulation is determined by the initial composition formulation provided that the mentioned conditions are fulfilled. A polymer in biostable and biocompatible implants can be only optically transparent, though the converse is not necessary.
- Buckhurst P.J., Naroo S.A., Shah S. Advanced intraocular lens designs. European Ophthalmic Review 2010; 4(1): 82–87.
- Richter-Mueksch S., Kahraman G., Amon M., Schild-Burggasser G., Schauersberger J., Abela-Formanek C. Uveal and capsular biocompatibility after implantation of sharp-edged hydrophilic acrylic, hydrophobic acrylic, and silicone intraocular lenses in eyes with pseudoexfoliation syndrome. J Cataract Refract Surg 2007; 33(8): 1414–1418.
- Pozdeeva N.A., Pashtaev N.P. Iskusstvennaya iridokhrustalikovaya diafragma v khirurgicheskom lechenii aniridii [Artificial iris-lens diaphragm in surgical treatment of aniridia]. Cheboksary; 2012; 160 p.
- Pozdeeva N.A. New model of artificial iris-lens diaphragm for correction of large iris defects (clinical and functional results of implantation). Vestnik oftal’mologii 2013; 129(6): 38–44.
- Iskakov I., Egorova E., Koronkevich V., Lenkova G., Korolkov V., Treushnikov V. Novel diffractive-refractive bifocal IOL: optical properties and earliest clinical results. In: XXIV Congress of the ESCRS (European Society of Cataract and Refractive Surgeons). London; 2006; p. 217.
- Pashtaev N.P., Pivovarov N.N., Treushnikov V.M., et al. Novaya model’ diafragmiruyushchey elastichnoy IOL. V kn.: Sovremennye tekhnologii kataraktal’noy i refraktsionnoy khirurgii — 2011 [A new model of diaphragm elastic IOL. In: Modern technologies of cataract and refractive surgery — 2011]. Moscow; 2011; p. 196–200.
- Maliugin B.E. State-of-the-art cataract surgery and intraocular optical correction. Vestnik oftal’mologii 2014; 130(6): 80–88.
- Trubilin V.N., Temirov N.N. Correction of aphakia various origins multifocal intraocular lenses with asymmetric rotational optics. Kataraktal’naya i refraktsionnaya khirurgiya 2014; 4: 20–25.
- Altynbaeva G.R. Osobennosti vybora mul'tifokal'nykh intraokulyarnykh linz v khirurgii katarakty. Avtoref. dis. ... kand. med. nauk [Peculiarities of choosing multifocal intraocular lens in cataract surgery. PhD Thesis]. Krasnoyarsk; 2012.
- Mencucci R., Favuzza E., Boccalini C., Gicquel JJ., Raimondi L. Square-edge intraocular lenses and epithelial lens cell proliferation: implications on posterior capsule opacification in an in vitro model. BMC Ophthalmol 2015; 15: 5, http://dx.doi.org/10.1186/1471-2415-15-5.
- Menapace R., Findl O., Kriechbaum K., Leydolt-Koeppl Ch. Accommodating intraocular lenses: a critical review of present and future concepts. Graefes Arch Clin Exp Ophthalmol 2007; 245(4): 473–489, http://dx.doi.org/10.1007/s00417-006-0391-6.
- Gutierrez L.G., Rodriguez P., Garcia D.A. Intraoperative opacification of a hydrophilic acrylic with hydrophobic surface IOL with spontaneous resolution in 24 hours. J Refract Surg 2013; 29(5): 360–362, http://dx.doi.org/10.3928/1081597X-20130313-03.
- Sobolev N.P., Malyugin B.E., Pokrovskiy D.F., Patakhova H.M. Surgical experience with angle-supported phakic IOLs for high myopia correction. Oftal’mokhirurgiya 2013; 4: 20–24.
- Vasavada A.R., Raj S.M., Shah A., Shah G., Vasavada V., Vasavada V. Comparison of posterior capsule opacification with hydrophobic acrylic and hydrophilic acrylic intraocular lenses. J Cataract Refract Surg 2011; 37(6): 1050–1059, http://dx.doi.org/10.1016/j.jcrs.2010.12.060.
- Maurino V., Allan B.D., Rubin G.S., Bunce C., Xing W., Findl O.; Moorfields IOL Study Group. Quality of vision after bilateral multifocal intraocular lens implantation: a randomized trial — AT LISA 809M versus AcrySof ReSTOR SN6AD1. Ophthalmology 2015; 122(4): 700–710.
- Nixon D.R., Woodcock M.G. Pattern of posterior capsule opacification models 2 years postoperatively with 2 single-piece acrylic intraocular lenses. J Cataract Refract Surg 2010; 36(6): 929–934, http://dx.doi.org/10.1016/j.jcrs.2009.12.040.
- Malyugin B.E., Takhtaev Y.V., Morozova T.A., Pozdeeva N.A. Clinical outcomes of the third generation multifocal gradient IOL implantation in prospective multicenter study. Oftal’mokhirurgiya 2012; 2: 36–41.
- Pashtaev N.P., Bat’kov E.N. The results of implantation of a new model of posterior chamber elastic IOL in insufficient capsular support. Oftal’mokhirurgiya 2009; 5: 34–39.
- Kuznetsov S.L., Uzunyan D.G., Zakhidov A.B., Novikov S.V., Selifanov Yu.V. IOL with torsion haptics. Clinical results of volume-substituting model study. Oftal’mokhirurgiya 2010; 2: 24–29.
- Hengerer F.H., Artal P., Kohnen T., Conrad-Hengerer I. Initial clinical results of a new telescopic IOL implanted in patients with dry age-related macular degeneration. J Refract Surg 2015 Mar; 31(3): 158–162, http://dx.doi.org/10.3928/1081597X-20150220-03.
- Calladine D., Evans J.R., Shah S., Leyland M. Мultifocal versus monofocal intraocular lenses after cataract extraction. Sao Paulo Med J 2015; 133(1): 68, http://dx.doi.org/10.1590/1516-3180.20151331T2.
- Kohnen T., Fabian E., Gerl R., Hunold W., Hütz W., Strobel J., Hoyer H., Mester U. Optic edge design as long-term factor for posterior capsular opacification rates. Ophthalmology 2008; 115(8): 1308–1314, http://dx.doi.org/10.1016/j.ophtha.2008.01.002.
- Malyugin B.E., Тereshchenko А.V., Belyy Yu.А., Demyanchenko S.К., Fadeyeva T.V., Isayev M.A. The comparative analysis of spherical and aspherical IOL implantation clinical efficacy. Oftal’mokhirurgiya 2011; 3: 27–31.
- Dawes L.J., Illingworth C.D., Wormstone I. A fully human in vitro capsular bag model to permit intraocular lens evaluation. Invest Ophthalmol Vis Sci 2012; 53(1): 23–29, http://dx.doi.org/10.1167/iovs.11-8851.
- Cleary G., Spalton D.J., Zhang J.J., Marshall J. In vitro lens capsule model for investigation of posterior capsule opacification. J Cataract Refract Surg 2010; 36(8): 1249–1252, http://dx.doi.org/10.1016/j.jcrs.2010.05.006.
- Ness P.J., Werner L., Maddula S., Davis D., Zaugg B., Stringham J., Burrow M., Yeh O. Pathology of 219 human cadaver eyes with 1-piece or 3-piece hydrophobic acrylic intraocular lenses: capsular bag opacification and sites of square-edged barrier breach. J Cataract Refract Surg 2011; 37(5): 923–930, http://dx.doi.org/10.1016/j.jcrs.2010.11.036.
- Roshdy M.M., Riad R.F., Morkos F.F., Hassouna A.K., Wahba S.S. Effect of a single-piece aspheric hydrophobic acrylic intraocular lens design on centration and rotation. J Cataract Refract Surg 2013; 39(3): 408–413, http://dx.doi.org/10.1016/j.jcrs.2012.09.020.
- Ghoreishi M., Agherian R., Peyman A.R., Feshareki H., Mohammadinia M. Flexible toric iris claw phakic intraocular lens implantation for myopia and astigmatism. J Ophthalmic Vis Res 2014; 9(2): 174–180.
- Nixon D.R., Apple D.J. Evaluation of lens epithelial cell migration in vivo at the haptic-optic junction of a one-piece hydrophobic acrylic intraocular lens. Am J Ophthalmol 2006; 142(4): 557–562, http://dx.doi.org/10.1016/j.ajo.2006.05.049.
- Raj S.M., Vasavada A.R., Kaid J.S., Vasavada V.A., Vasavada V.A. Post-operative capsular pacification. Nepal J Ophthalmol 2009; 1(1): 43–59, http://dx.doi.org/10.3126/nepjoph.v1i1.3673.
- Gushchina M.B., Treushnikov V.V., Sorokina O.V. Skleroplantat dlya rekonstruktivnoy skleroplastiki pri patologicheskikh sostoyaniyakh sklery [Scleroplant for reconstructive scleroplasty in sclera pathologies]. RU patent 2460497. 2010.
- Anisimova S.Yu., Anisimov S.I., Drozdova G.A., Larionov E.V., Ozornina O.S. Results of use of scleroplastic material on the xenocollagen basis in progressing myopia treatment. Rossiyskaya pediatricheskaya oftal’mologiya 2009; 3: 35–38.
- Iomdina E.N. Biomekhanika skleral’noy obolochki glaza pri miopii: diagnostika narusheniy i ikh eksperimental’naya korrektsiya. Avtoref. dis. … dokt. biol. nauk [Biomechanics of sclera membrane in myopia: diagnostics of impairments and their experimental correction. DSc Thesis]. Moscow; 2000.
- Rada J.A., Shelton S., Norton T.T. The sclera and myopia. Exp Eye Res 2006; 82(2): 185–210, http://dx.doi.org/10.1016/j.exer.2005.08.009.
- Kuznetsova M.V. Prichiny razvitiya blizorukosti i ee lechenie [Etiology of myopia and its treatment]. Moscow: MEDpress-inform; 2005; 176 p.
- Filatova G.P. Implantatsiya biologicheskikh materialov pri skleroukreplyayushchikh operatsiyakh (eksperimental’no-klinicheskoe issledovanie). Avtoref. dis. … kand. med. nauk [Implantation of biological materials in sclera restorative surgeries (experimental and clinical study). PhD Thesis]. Moscow; 2009.
- Neroev V.V., Tarutta E.P., Oganesyan O.G., Penkina A.V., Khandzhyan A.T., Milash S.V. Assessment of the impact of intrastromal corneal segment implantation (Ferrara ring) on the parameters of anterior and posterior corneal curvature using a scheimpflug analyzer Galilei G2. Novoe v oftal’mologii 2014; 2: 60–62.
- Lam K., Rootman D.B., Lichtinger A., Rootman D.S. Post-LASIK ectasia treated with intrastromal corneal ring segments and corneal crosslinking. Digit J Ophthalmol 2013; 19(1): 1–5.
- Jadidi K., Mosavi S.A., Nejat F., Naderi M., Janani L., Serahati S. Intrastromal corneal ring segment implantation (keraring 355°) in patients with central keratoconus: 6-month follow-up. J Ophthalmol 2015; 2015: 916385, http://dx.doi.org/10.1155/2015/916385.
- Weber C.H., Cionni R.J. All about capsular tension rings. Curr Opin Ophthalmol 2015; 26(1): 10–15, http://dx.doi.org/10.1097/ICU.0000000000000118.
- Wilkie D.A., Stone Hoy S., Gemensky-Metzler A., Colitz C.M. Safety study of capsular tension ring use in canine phacoemulsification and IOL implantation. Vet Ophthalmol 2014, http://dx.doi.org/10.1111/vop.12232. [Epub ahead of print].
- Biró Z., Szabó I., Pámer Z. Combined cataract surgery on a Marfan-syndrome patient (case report). Oftalmologia 2014; 58(2): 30–33.
- Rodrigo B.J., Paulina L.L., Francesc Mde R., Eduardo T.T., Alejandro N. Intraocular lens subluxation in Marfan syndrome. Open Ophthalmol J 2014; 8: 48–50, http://dx.doi.org/10.2174/1874364101408010048.
- Kuznetsov S.L. Vliyanie vnutrikapsul’nogo stabiliziruyushchego kol’tsa na polozhenie intraokulyarnykh linz s ploskostnoy gaptikoy (predvaritel’noe soobshchenie). V kn.: Eroshevskie chteniya: trudy Vserossiyskoy konferentsii [The effect of an intracapsular stabilizing ring on the position of intraocular lens with planar haptics (a preliminary report). In: Eroshevsky readings: proceedings of All-Russia conference]. Samara; 2007; p. 226–229.
- Kuznetsov S.L. Rezul’taty izucheniya endokapsulyarnykh korrelyatsiy pri implantatsii vnutrikapsul’nykh kolets i IOL s ploskostnoy gaptikoy v eksperimente. V kn.: Sovremennye tekhnologii khirurgii katarakty: materialy 5-y Mezhdunarodnoy nauchno-prakticheskoy konferentsii [Results of studying endocapsular correlations when implanting intracapsular rings and IOL with planar haptics in experiment. In: Modern technologies of cataract surgery: proceedings of 5th International research and practice conference]. Moscow; 2004; p. 188–193.
- Kuznetsov S.L. Rezul’taty implantatsii vnutrikapsul’nykh kolets iz polipropilenovykh nitey v kachestve sredstva dopolnitel’noy fiksatsii IOL s ploskostnoy gaptikoy v eksperimente. V kn.: Glaukoma i drugie problemy oftal’mologii: sbornik nauchnykh trudov, posvyashchennyy 15-letiyu Tambovskogo filiala GU MNTK “MG” im. akademika S.N. Fedorova [Implantation outcomes of intracapsular rings of polypropylene fibers as an adjunctive fixation means for IOL with planar haptics in experiment. In: Glaukoma and other ophthalmological problems: collection of scientific papers devoted to 15th anniversary of Tambov branch of State Institution “Interbranch Scientific and Technical Complex “Eye Microsurgery” named after Academician S.N. Fedorov]. Tambov; 2005; p. 221–227.
- Fankhauser F. Microincision IOL outcomes positive after a year. EuroTimes 2006; 11(9): 9.
- Kuznetsov S.L. Results of experimental study of endocapsular correlations of various intracapsular ring models and plate-haptic IOLs. In: XXIV Congress of the ESCRS: abstracts. London; 2006; p. 229.
- Burger J., Kreutzer T., Alge C.S., Strauss R.W., Eibl K., Haritoglou C., Neubauer A.S., Kampik A., Priglinger S.G. Capsular tension ring-based in vitro capsule opacification model. J Cataract Refract Surg 2008; 34(7): 1167–1172, http://dx.doi.org/10.1016/j.jcrs.2008.03.040.
- Egorova E.V., Betke A.V., Bezborodov V.G. Mathematical modeling in a solution of the long-term complications problem of cataract surgery with zonular weakness. Oftal’mokhirurgiya 2014; 3: 13–18.
- Grinev A.G. Sviridova M.B., Herebtsova O.M., Dolgopolova M.S. Clinical cases of cataract phacoemulsification in eye with small pupil, big hard nuclei and lens subluxation. Ural’skiy meditsinskiy zhurnal 2013; 9: 103–105.
- Ioshin I.E. Intracapsular ring in cataract surgery in lens subluxation (15 years experience). Vestnik oftal’mologii 2012; 128(2): 45–49.
- Malyugin B.E. Malyugin ring. Novoe v oftal’mologii 2013; 4: 59–61.
- Nizov A.V., Stepanov A.V. The efficiency of Ahmed valve in posttraumatic glaucoma surgery. Kataraktal’naya i refraktsionnaya khirurgiya. 2011; 11(4): 52–54.
- Tereshchenko A.V., Molotkova I.A., Belyy Yu.A., Erokhina E.V. Modification of the modern microinvasive non-penetrating glaucoma surgery with use of T-shaped drainage. Oftal’mokhirurgiya 2011; 2: 38–42.
- Galassi F., Giambene B. Deep sclerectomy with SkGel implant: 5-year results. J Glaucoma 2008; 17(1): 52–56, http://dx.doi.org/10.1097/IJG.0b013e3180d0a885.
- Bikbov M.M., Babushkin A.E., Chaika O.V., Orenburkina O.I., Matiukhina E.N. Results of fistulizing and Ahmed valve surgery for treatment of refractory glaucoma. Vestnik oftal’mologii 2014; 130(2): 8–11.
- Vinod K., Frolov M.A., Bozhok E.V., Dushina G.N. Experience in the application of metal drainage of domestic construction in glaucoma surgery. Novoe v oftal’mologii 2012; 4: 43–45.
- Figus M., Lazzeri S., Fogagnolo P., Iester M., Martinelli P., Nardi M. Supraciliary shunt in refractory glaucoma. Br J Ophthalmol 2011; 95(111): 1537–1541, http://dx.doi.org/10.1136/bjophthalmol-2011-300308.
- Ryazantseva T.V., Kravets L.I. Explantodrainage with nanostructured surface for refractory glaucoma surgery. Byulleten’ sibirskoy meditsiny 2012; 11(1): 71–76.
- Pozdeyeva N.A., Gorbunova N.Y., Pashtayev N.P. Efficacy of valve drainage devices in secondary glaucoma in patients with artificial iridolenticular diaphragm. Vestnik oftal’mologii 2011; 127(4): 41–45.
- Yevstigneyeva Yu.V. Collagen implant in refractory glaucoma surgery. Vestnik oftal’mologii 2011; 127(1): 36–38.
- Takhchidi Kh.P., Cheglakov V.Yu. Drainages in refractory glaucoma surgery. Refraktsionnaya khirurgiya i oftal’mologiya 2009; 3: 11–15.
- Neroev V.V., Bykov V.P., Kvasha O.I., Belevtseva T.A. Micro draining surgery in glaucoma treatment. Literary review. Russkiy meditsinskiy zhurnal 2009; 3: 113–116.
- Wang H., Dong H., Kang C.G., Lin C., Ye X., Zhao Y.L. Preliminary exploration of the development of a collagenous artificial dura mater for sustained antibiotic release. Chin Med J (Engl) 2013; 126(17): 3329–3333.
- Lv C., Zhou Z., Song Y., Liu L., Liu H., Gong Q., Li T., Zeng J., Tu C., Pei F. Novel biodegradable lamina for lamina repair and reconstruction. Spine J 2013; 13(12): 1912–1920, http://dx.doi.org/10.1016/j.spinee.2013.06.055.
- Bai W., Wang X., Yuan W., Wang H., Wang Z. Application of PLGA/type I collagen/chitosan artificial composite dura mater in the treatment of dural injury. J Mater Sci Mater Med 2013; 24(9): 2247–2254, http://dx.doi.org/10.1007/s10856-013-4964-8.
- Tikhomirov S.E., Tsybusov S.N., Kravets L.Ya., Fraerman A.P., Balmasov A.A. Plasty of the base of the skull defects and dura mater with the reperen’s new polymer material. Sovremennye tehnologii v medicine 2010; 2: 6–11.
- Shesterickov A.A., Lalov Yu.V., Fomin P.A., Uspensky I.V. Hermetization of the turkish saddle fundus with the “Reperen-ST” synthetic implant in a combined treatment of the chiasmal and sellar area tumors. Sovremennye tehnologii v medicine 2011; 1: 6–10.
- Ivanov S.Y., Zaitsev A.B., Yamurkova N.F., Migura S.A., Gubova V.M., Yantsen I.E., Akulov M.M., Muraev A.A. The study of barrier function of collagen membrane “Osteoplast” in healing bone defects in an experiment. Sovremennye tehnologii v medicine 2011; 3: 35–38.
- Matsumoto Y., Aikawa H., Tsutsumi M., Narita S., Yoshida H., Etou H., Sakamoto K., Kazekawa K. Histological examination of expanded polytetrafluoroethylene artificial dura mater at 14 years after craniotomy: case report. Neurol Med Chir (Tokyo) 2013; 53(1): 43–46, http://dx.doi.org/10.2176/nmc.53.43.
- Uspenskiy I.V., Treushnikov V.V., Sorokina O.V., Tikhomirov S.E., Fraerman A.P., Kravets L.Ya. Implantat dlya plastiki defektov tverdoy mozgovoy obolochki [An implant for dura mater defect plasty]. RU patent 2436596. 2009.
- Matsumoto Y., Aikawa H., Tsutsumi M., Narita S., Yoshida H., Etou H., Sakamoto K., Kazekawa K. Histological examination of expanded polytetrafluoroethylene artificial dura mater at 14 years after craniotomy: case report. Neurol Med Chir (Tokyo) 2013; 53(1): 43–46, http://dx.doi.org/10.2176/nmc.53.43.
- Christoffersen M.W., Brandt E., Helgstrand F., Westen M., Rosenberg J., Kehlet H., Strandfelt P., Bisgaard T. Recurrence rate after absorbable tack fixation of mesh in laparoscopic incisional hernia repair. Br J Surg 2015; 102(5): 541–547, http://dx.doi.org/10.1002/bjs.9750.
- Li J., Ji Z., Zhang W., Li L. The comparison of lightweight mesh and standard mesh in incisional hernia repair with the open sublay technique: the results of a meta-analysis. Surg Laparosc Endosc Percutan Tech 2015; 25(3): 238–244, http://dx.doi.org/10.1097/SLE.0000000000000144.
- Parshikov V.V., Medvedev A.P., Samsonov A.A., Romanov R.V., Samsonov A.V., Gradusov V.P., Petrov V.V., Khodak V.A., Baburin A.B. Tension-free plasty in the surgery of abdominal wall hernias. Vestnik hirurgii im. I.I. Grekova 2010; 169(5): 74–79.
- Khodak V.А., Petrov V.V., Dvornikov А.V., Mironov А.А., Baburin А.B., Parshikov V.V., Tsybusov S.N. The possibilities and advantages of sutureless plasty of abdominal wall using different synthetic meshes in experimental study. Sovremennye tehnologii v medicine 2012; 2: 31–36.
- Sedov V.M., Gostevskoy A.A., Tarbaev S.D., Gorelov A.S., Chulkhovin A.V., Nutfullina G.M., Zhukovsky V. Meshed implants of polyvinylidene fluoride in treatment of abdominal wall hernias. Vestnik hirurgii im. I.I. Grekova 2008; 167(2): 16–21.
- Rehman S., Khan S., Pervaiz A., Perry E.P. Recurrence of inguinal herniae following removal of infected prosthetic meshes: a review of the literature. Hernia 2012; 16(2): 123–126, http://dx.doi.org/10.1007/s10029-011-0873-2.
- Fedorov I.V. Prostheses in hernia surgery: the hundred years evolution. Novyy khirurgicheskiy arkhiv 2002; 4(1).
- Berthet J.P., Canaud L., D’Annoville T. Titanium plates and Dualmesh: a modern combination for reconstructing very large chest wall defects. Ann Thorac Surg 2011; 91(6): 1709–1716, http://dx.doi.org/10.1016/j.athoracsur.2011.02.014.
- Zhukovskiy V.A. Polimernye endoprotezy dlya gernioplastiki [Polymer endoprostheses for hernioplasty]. Saint Petersburg: Eskulap; 2011; 104 p.
- Averyanov M.Y., Gaar E.V., Gorokhov V.N. Comparative analysis of the use of non-tension and traditional hernioplasty techniques in abdominal hernias of various localizations. Sovremennye tehnologii v medicine 2011; 3: 39–43.
- Kouhia S., Vironen J., Hakala T., Paajanen H. Open mesh repair for inguinal hernia is safer than laparoscopic repair or open non-mesh repair: a nationwide registry study of complications. World J Surg 2015, http://dx.doi.org/10.1007/s00268-015-3028-2. [Epub ahead of print].
- Descloux A., Pohle S., Nocito A., Keerl A. Hybrid NOTES transvaginal intraperitoneal onlay mesh in abdominal wall hernias: an alternative to traditional laparoscopic procedures. Surg Endosc 2015, http://dx.doi.org/10.1007/s00464-015-4141-x. [Epub ahead of print].
- Kathju S., Nistico L., Melton-Kreft R., Lasko L.A., Stoodley P. Direct demonstration of bacterial biofilms on prosthetic mesh after ventral herniorrhaphy. Surg Infect (Larchmt) 2015; 16(1): 45–53, http://dx.doi.org/10.1089/sur.2014.026.
- Christmas A.B., Honaker D. Incarcerated massive sliding hernia treated with bladder resection and mesh repair. Am Surg 2015; 81(3): 123–124.
- Salokorpi N., Sinikumpu J.J., Iber T., Zibo H.N., Areda T., Ylikontiola L., Sándor G.K., Serlo W. Frontal cranial modeling using endocranial resorbable plate fixation in 27 consecutive plagiocephaly and trigonocephaly patients. Childs Nerv Syst 2015, http://dx.doi.org/10.1007/s00381-015-2657-y. [Epub ahead of print].
- Liebelt B.D., Huang M., Baskin D.S. Sellar floor reconstruction with the Medpor® implant versus autologous bone following transnasal transsphenoidal surgery: outcome in 200 consecutive cases. World Neurosurg 2015, http://dx.doi.org/10.1016/j.wneu.2015.02.025. [Epub ahead of print].
- Durnovo E.A., Khomutinnikova N.E., Mishina N.V., Trofimov A.O. The peculiarities of the reconstruction of the walls of orbital cavity during the treatment of traumatic damages of facial skeleton. Meditsinskiy al’manakh 2013; 5: 159–161.
- Piitulainen J.M., Kauko T., Aitasalo K.M., Vuorinen V., Vallittu P.K., Posti J.P. Outcomes of cranioplasty with synthetic materials and autologous bone grafts. World Neurosurg 2015; 83(5): 708–714, http://dx.doi.org/10.1016/j.wneu.2015.01.014.
- Chaya A., Yoshizawa S., Verdelis K., Myers N., Costello B.J., Chou D.T., Pal S., Maiti S., Kumta P.N., Sfeir C. In vivo study of magnesium plate and screw degradation and bone fracture healing. Acta Biomater 2015; 18: 262–269, http://dx.doi.org/10.1016/j.actbio.2015.02.010.
- Kutikov A.B., Skelly J.D., Ayers D.C., Song J. Templated repair of long bone defects in rats with bioactive spiral-wrapped electrospun amphiphilic polymer/hydroxyapatite scaffolds. ACS Appl Mater Interfaces 2015; 7(8): 4890–4901, http://dx.doi.org/10.1021/am508984y.
- Lu T., Wen J., Qian S., Cao H., Ning C., Pan X., Jiang X., Liu X., Chu P.K. Enhanced osteointegration on tantalum-implanted polyetheretherketone surface with bone-like elastic modulus. Biomaterials 2015; 51: 173–183, http://dx.doi.org/10.1016/j.biomaterials.2015.02.018.
- Kim I.G., Hwang M.P., Du P., Ko J., Ha C.W., Do S.H., Park K. Bioactive cell-derived matrices combined with polymer mesh scaffold for osteogenesis and bone healing. Biomaterials 2015; 50: 75–86, http://dx.doi.org/10.1016/j.biomaterials.2015.01.054.
- Hinderer S., Shena N., Ringuette L.J., Hansmann J., Reinhardt D.P., Brucker S.Y., Davis E.C., Schenke-Layland K. In vitro elastogenesis: instructing human vascular smooth muscle cells to generate an elastic fiber-containing extracellular matrix scaffold. Biomed Mater 2015; 10(3), http://dx.doi.org/10.1088/1748-6041/10/3/034102.
- Orłowska J., Kurczewska U., Derwińska K., Orłowski W., Orszulak-Michalak D. The use of biodegradable polymers in design of cellular scaffolds. Postepy Hig Med Dosw (Online) 2015 Mar; 69: 294–301, http://dx.doi.org/10.5604/17322693.1142717.
- Biosovmestimye materialy [Biocompatible materials]. Pod red. Sevast’yanova V.I., Kirpichnikova M.P. [Sevast’yanova V.I., Kirpichnikov M.P. (editors)]. Moscow: Med. informatsionnoe agentstvo; 2011; 540 p.
- Gumargalieva K.Z., Zaikov G.E., Moiseev Yu.V. Macrokinetic aspects of polymer biocompatibility and biodegradability. Uspekhi khimii 1994; 63(10): 905–921.
- Khench L., Dzhons D. Biomaterialy, iskusstvennye organy i inzhiniring tkaney. Seriya “Mir biologii i meditsiny” [Biomaterials, artificial organs and tissue engineering. Issue “World of biology and medicine”]. Moscow: Tekhnosfera; 2007; 304 p.
- Korzhikov V.A., Vlakh E.G., Tennikova T.B. Polymers in orthopedic surgery and tissue engineering: from engineering materials to smart biofunctionalization of a surface. Polymer Science Series A 2012; 54(8): 1203–1221, http://dx.doi.org/10.1134/s0965545x12070036.
- Rikli D.A., Curtis R., Schilling C., Goldhahn J. Application potential of biodegradable plates and screws for treatment of distal radial bone fractures. Margo Anterior 2002; 4: 1–4.
- Vallet-Regi M., Colilla M., González B. Medical applications of organic-inorganic hybrid materials within the field of silica-based bioceramics. Chem Soc Rev 2011; 40(2): 596–607, http://dx.doi.org/10.1039/c0cs00025f.
- Treushnikov V.M. Basic principles of biocompatible implant manufacturing. Nizhegorodskie vedomosti meditsiny 2007; 6: 46–55.
- Valuev L.I., Davydov D.V., Sytov G.A., Valuev I.L. Hydrogel ophthalmic implants. Polymer Science Series A 2014; 56(6): 786–788, http://dx.doi.org/10.1134/S0965545X1406011X.
- Treushnikov V.M., Viktorova A. Basic principles of biocompatible implant manufacturing. In: International Symposium “New polymers and radioprotectors for biology and medicine”. Yerevan, Armenia, 8–10 October, 2007.
- Duan Yuan-yuan, Jia Jun, Wang Shao-hai, Yan Wei, Jun Lei, Wang Zhong-yi. Preparation of PLGA electrospun nanofibers for tissue engineering applications. Journal of US-China Medical Science 2007; 4(1, Serial 26): 41–44.
- Ito Y., Hasuda H., Kamitakahara M., Ohtsuki C., Tanihara M., Kang I.K., Kwon O.H. A composite of hydroxyapatite with electrospun biodegradable nanofibers as tissue engineering. J Biosci Bioeng 2005; 100(1): 43–49, http://dx.doi.org/10.1263/jbb.100.43.
- Li M., Mondrinos M.J., Gandhi M.R., Ko F.K., Weiss A.S., Lelkes P.I. Electrospun protein fibers as matrices for tissue engineering. Biomaterials 2005; 26(30): 5999–6008, http://dx.doi.org/10.1016/j.biomaterials.2005.03.030.
- Vasilets V.N., Kazbanov I.V., Efimov A.E., Sevastianov V.I. New methods for implant matrix formation based on electrospinning and bioprinting technologies. Vestnik transplantologii i iskusstvennykh organov 2009; 11(2): 47–53.
- Sevastianov V.I. Biomaterials, drug delivery systems, and bioengineering. Vestnik transplantologii i iskusstvennykh organov 2009; 11(3): 69–80.
- Sevastianov V.I., Vasilets V.N., Agapov I.I. Biopolymer implants for high-technology assistance in the field of replacement and regenerative medicine. Rare metals 2009; 28: 84–86.
- Bazhenov S.L., Berlin A.A., Kul’kov A.A., Oshmyan V.G. Polimernye kompozitsionnye materialy. Prochnost’ i tekhnologii [Polymer composition materials. Strength and technologies]. Moscow: Intellekt; 2009; 352 p.
- Perepelkin K.E. Armiruyushchie volokna i voloknistye polimernye kompozity [Reinforcing fibers and fibrous polymer composites]. Moscow: Nauchnye osnovy i tekhnologii; 2009; 658 p.
- Zolotareva N.V., Semenov V.V., Myakov V.N., Kulikova T.I., Arapova A.V., Faerman V.I., Gorshkov O.N., Kasatkin A.P., Kotomina V.E., Kruglov A.V., Trushin V.N., Treushnikov V.V., Treushnikov V.M. Formation of microchannes in heat-curable silicone rubber using filamentary crystals of p-aminobenzoic acid. Izvestiya Akademii nauk. Seriya khimicheskaya 2015; 1: 189–195.
- Wu Y., Dudek S.T., Bamgbade B.A., McHugh M.A. High-pressure phase behavior of boltorn hyperbranched polymers in supercritical fluids. Fluid Phase Equilibria 2014; 382: 180–186, http://dx.doi.org/10.1016/j.fluid.2014.09.010.
- Popov V.K., Krasnov A.P., Volozhin A.I., Houdl S.M. New bioactive composite materials for bone tissues regeneration. Perspektivnye materialy 2004; 4: 49–57.
- Mironova L.A. Acrylic basis with Сalcium-MAKG supplement. Rossiyskaya stomatologiya 2013; 1: 25–27.
- Tkachenko V.M. Razrabotka osteointegrativnogo gelya gialuronovaya kislota gidroksiappatit s bakteritsidnymi svoystvami. V kn.: Bolezni tsivilizatsii v aspekte ucheniya V.I. Vernadskogo: materialy 3-y Mezhdunarodnoy konferentsii [Development of osteointegrative gel: hyaluronic acid hydroxylapatite with antibacterial properties. In: Civilization diseases in the terms of V.I. Vernadsky theory: proceedings of 3rd International conference]. Moscow; 2005; p. 317.
- Antonov E.N., Bagratashvili V.N., Whitaker M.J., Barry J.J., Shakesheff K.M., Konovalov A.N., Popov V.K., Howdle S.M. Three-dimensional bioactive and biodegradable scaffolds fabricated by laser sintering. Adv Mat 2005; 17(3): 327–330, http://dx.doi.org/10.1002/adma.200400838.
- Antonov E.N., Bagratashvili V.N., Howdle S.M., Konovalov A.N., Popov V.K., Panchenko V.Ya. Fabrication of polymer scaffolds for tissue engineering using surface selective laser sintering. Laser Physics 2006; 16(5): 774–787, http://dx.doi.org/10.1134/s1054660x06050070.
- Kanczler J.M., Mirmalek-Sani S.H., Hanley N.A., Ivanov A.L., Barry J.J., Upton C., Shakesheff K.M., Howdle S.M., Antonov E.N., Bagratashvili V.N., Popov V.K., Oreffo R.O. Biocompatibility and osteogenic potential of human fetal femur-derived cells on surface selective laser sintered scaffolds. Acta Biomaterialia 2009; 5(6): 2063–2071, http://dx.doi.org/10.1016/j.actbio.2009.03.010.
- Panchenko V.Ya. Lazerno-informatsionnye tekhnologii: sostoyanie del, proekty. V kn.: Puti uchenogo. E.P. Velikhov [Laser information technologies: status, projects. In: Career of a scientist: Е.P. Velikhov]. Pod red. Smirnova V.P. [Smirnov V.P. (editor)]. Moscow: TIzd-vo NITs “Kurchatovskiy institut”; 2007; p. 293–295.
- Varadan V., Vinoy K., Dzhoze K. Mir elektroniki VCh MEMS i ikh primenenie [RF MEMS electronics community and their application]. Moscow: Tekhnosfera; 2004; 528 p.
- Chesnokov S.A. Polimerizatsiya monomerov (met)akrilovogo ryada pod deystviem vidimogo sveta, initsiiruemaya o-khinonami. Avtoref. dis. …. dokt. khim. nauk [Polymerization of monomers of (meth)acrylic series under exposure to visible light initiated by о-quinones. DSc Thesis]. Nizhny Novgorod; 2014.
- Owen S.C., Shoichet M.S. Design of three-dimensional biomimetic scaffolds. J Biomed Mater Res A 2010; 94(4): 1321–1331, http://dx.doi.org/10.1002/jbm.a.32834.
- Everland H., Samuelsen P., Vange J., Clausen C., Gallego M.R. Compositions and methods for augmentation and regeneration of living tissue in a subject. US patent 8,877,246. 2010.
- Baer Hans U. Matrix and implant for tissue engineering. WO 2014202199. 2014.
- Rosbach J., Choritz L., Pfeiffer N., Thieme H. Clinical results of encapsulated bleb removal after Ahmed glaucoma valve implants. Dert Ophthalmologe 2013; 110(8): 722–727, http://dx.doi.org/10.1007/s00347-013-2836-8.
- Viakh E.G., Korzhikov V.A., Tennikova T.B. Solid-phase systems of biological recognition based on macroporous polymer monoliths. Izvestiya Akademii nauk. Seriya khimicheskaya 2012; 5: 931–956.
- Mukhina I.V., Tsybusov S.N., Vedunova M.V., Trifonova A.S., Treushnikov V.M., Kolmogorov Yu.N., Treushnikov V.V., Sorokina O.V. Matritsa dlya kletochnoy transplantologii [Matrix for cell transplantology]. RU patent 2521194. 2014.
- Shlyapintokh E.Ya. Fotokhimicheskie prevrashcheniya i stabilizatsiya polimerov [Photochemical transformation and stabilization of polymers]. Moscow: Khimiya; 1979; 344 p.
- Emanuel’ N.M., Buchachenko A.L. Khimicheskaya fizika molekulyarnogo razrusheniya i stabilizatsii polimerov [Chemical physics of molecular destruction and stabilization of polymers]. Moscow: Nauka; 1988; 366 p.
- Treushnikov V.M., Chesnokov S.A. Single-stage processes of polymer products photochemical synthesis with optical accuracy. Journal of Photochemistry and Photobiology A: Chemistry 2008; 196:201–209, http://dx.doi.org/10.1016/j.jphotochem.2007.07.030.
- Chesnokov S.A., Chechet Yu.V., Cherkasov V.K., Mamysheva O.N., Treushnikov V.M. General conditions and experimental design of sustained frontal photopolymerization in photopolymerizable liquid compositions. Polymer Science. Series A 2008; 50(3): 291–298, http://dx.doi.org/10.1134/s0965545x08030073.
- Feller V. Vvedenie v teoriyu veroyatnostey i ee prilozheniya. T. 1 [An introduction to probability theory and its applications. Vol. 1]. Moscow: Mir; 1967; 498 p.
- Treushnikov V.M., Pyatygin S.S., Opritov V.A. Interpretations of “critical” phenomena in the work of membrane bound enzyme systems based on a continual diffusion model. Biologicheskie membrany 1991; 8(10): 1093–1098.
- Treushnikov V.M., Pyatygin S.S., Opritov V.A. Application of the continual diffusion model for analysis of the principles of enzymatic reaction rate regulation under membrane conditions. Membrane and Cell Biology 1995; 8(4): 435–446.
- Pyatygin S.S., Treushnikov V.M., Opritov V.A., Krauz V.O. The phenomenon of negative temperature dependence of adaptive repolarization of high plant cells when exposed to cold. Fiziologiya rasteniy 1996; 43(1): 80–86.
- Treushnikov V.M., Pyatygin S.S., Opritov V.A., Orlova O.V. The phenomenon of negative temperature dependence of enzymatic reactions and its functional role. Vestnik Nizhegorodskogo universiteta im. N.I. Lobachevskogo. Seriya Biologiya 2001; 1(2): 198–207.
- Treushnikov V.M., Pomerantseva L.L., Zelentsova N.V., Oleynik A.V. O vozmozhnykh putyakh prevrashcheniy kvaziustoychivykh radikal’nykh tsentrov, obrazuyushchikhsya pri fotolize aromaticheskikh azidov v polimernykh matritsakh [Concerning possible transformation pathways of quasi-stable radical centers resulting from photolysis of aromatic azides in polymeric matrices]. Vysokomolekulyarnye soedineniya. Seriya B 1983; 25(5): 327–331.
- Semenov N.N. Tsepnye reaktsii [Chain reaction]. Moscow: Nauka; 1986; 535 p.
- Gladyshev G.P., Popov V.A. Radikal’naya polimerizatsiya pri glubokikh stepenyakh prevrashcheniya [Radical polymerization in deep transformations]. Moscow: Nauka; 1974; 243 p.
- Semchikov Yu.D. Vysokomolekulyarnye soedineniya [High-molecular connections]. Moscow: Akademiya; 2008; 367 p.
- Berlin A.A., Kefeli T.Ya., Korolev G.V. Poliefirakrilaty [Polyester acrylate]. Moscow: Nauka; 1967; 374 p.
- Berlin A.A., Korolev G.V., Kefeli T.Ya., Severgin Ya.M. Akrilovye oligomery i materialy na ikh osnove [Acrylic oligomers and materials on their base]. Moscow: Khimiya; 1983; 238 p.
- Korolev G.V., Mogilevich M.M., Il’in A.A. Assotsiatsiya zhidkikh organicheskikh soedineniy [Association of liquid organic compounds]. Moscow: Mir; 2002; 264 p.
- Grishin D.F., Semyonycheva L.L. Problems of control of the reactivity of macroradicals and the growth of polymer chains. Russian Chemical Reviews 2001; 70(5): 425–447, http://dx.doi.org/10.1070/RC2001v070n05ABEH000635.
- Kaush G. Razrushenie polimerov [Destruction of polymers]. Moscow: Mir; 1981; 440 p.
- Butyagin P.Yu., Dubinskaya A.M., Radtsig V.A. Electron spin resonance spectra, conformation, and chemical properties of free radicals in solid polymers. Russian Chemical Reviews 1969; 38(4): 290–305, http://dx.doi.org/10.1070/RC1969v038n04ABEH001742.
- Fedorov S.N., Linnik L.F., Treushnikov V.M., Viktorova E.A. Elastichnyy iskusstvennyy khrustalik i sposob ego izgotovleniya [Flexible artificial lens and its fabrication method]. RU patent 2074673. 1995.
- Fedorov S.N., Linnik L.F., Treushnikov V.M., Viktorova E.A., Karavaev A.A. Elastichnyy iskusstvennyy khrustalik glaza [Flexible artificial eye lens]. RU patent 2129880. 1999.
- Treushnikov V.M., Zueva T.A., Esin S.A., Oleynik A.V. Zhurnal nauchnoy i prikladnoy fotografii i kinematografii 1990; 34(3): 167–172.
- Treushnikov V.M., Esin S.A., Zueva T.A., Semchikov Yu.D., Knyazeva T.E., Yanin A.M., Semenova O.M. Kinetic features of radical polymerization in thin layers of photopolimerizable compositions. Vysokomolekulyarnye soedineniya. Seriya A 1995; 37(12): 1191–1197.
- Frenkel' Ya.I. Kineticheskaya teoriya zhidkosti [Kinetic theory of liquid]. Moscow: Nauka; 1975; 592 p.
- Fedorov S.N., Linnik L.F., Treushnikov V.M., Viktorova E.A., Karavaev A.A. Sposob izgotovleniya elastichnykh iskusstvennykh khrustalikov glaza [A fabrication method of flexible artificial eye lens]. RU patent 2129846. 1999.
- Treushnikov V.M., Viktorova E.A. Sposob izgotovleniya elastichnykh iskusstvennykh khrustalikov glaza [A fabrication method of flexible artificial eye lens]. RU patent 2198630. 2003.
- Treushnikov V.M., Viktorova E.A. Sposob izgotovleniya elastichnykh iskusstvennykh khrustalikov glaza [A fabrication method of flexible artificial eye lens]. RU patent 2234417. 2004.
- Klapshina L.G., Douglas W.E., Grigoryev I.S., Korytin A.I., Lavrentiev S.A., Lopatin M.A., et al. Novel metal-template assembled highly-functionalized cyanoporphyrazine ytterbium and vanadium complexes for potential photonic and optoelectronic applications. J Mater Chem 2009; 19(22): 3668–3676, http://dx.doi.org/10.1039/b821667c.
- Domrachev G.A., Semenov V.V., Klapshina L.G., Baten’Kin M.A., Arapova A.V., Kirillov A.I., Lopatin M.A., Obyedkov A.M., Zolotareva N.V., Gorshkov O.N., Kasatkin A.P., Mikhailov A.N., Antonov I.N., Sidorenko K.V., Treushnikov V.M., Treushnikov V.V. The light-emitting and optical properties of high-optical-quality organic glasses doped with europium tris(benzoyltrifluoroacetonate). Nanotechnologies in Russia 2009; 4(3–4): 225–236, http://dx.doi.org/10.1134/s1995078009030100.
- Semenov V.V., Zolotareva N.V., Lopatin M.A., Faerman V.I., Domrachev G.A., Gorshkov O.N., Kasatkin A.P., Skamnitskii D.V., Shenina M.E., Kruglov A.V., Treushnikov V.M., Treushnikov V.V. Spectral and optical properties of high-optical-quality organic glasses under prolonged ultraviolet irradiation. Polymer Science. Series A 2010; 52(6): 599–609, http://dx.doi.org/10.1134/s0965545x10060052.
- Grigoryev I.S., Klapshina L.G., Lermontova S.A., Semenov V.V., Treushnikov V.M., Treushnikov V.V., Bushuk B.A., Clement S., Douglas W.E. Efficient luminescent solar concentrators based on defectless organic glasses containing novel ytterbium cyanoporphyrazine complex. Nanotechnologies in Russia 2012; 7(9–10): 492–498, http://dx.doi.org/10.1134/s1995078012050059.
- Molodnyakov S.P., Treushnikov V.V., Treushnikov V.M., Gorshkov O.N., Kasatkin A.P., Shenina M.E., Shushunov A.N., Kruglov A.V., Semenov V.V. Polymeric waveguides based on photopolymerizing methacrylate compositions. Russian Journal of Applied Chemistry 2014; 87(3): 331–335, http://dx.doi.org/10.1134/S1070427214030148.
- Pashtaev N.P., Pozdeeva H.A., Starostina O.V., Morozova V.H. Iridokhrustalikovaya diafragma i sposob ee izgotovleniya [Iris-lens diaphragm and the process of its manufacturing]. RU patent 2526245. 2013.
- Tsybusov S.N., Durnovo E.A., Khomutinnikova N.E., Treushnikov V.M., Viktorova E.A., Treushnikov V.V., Sorokina O.V. Matritsa dlya regeneratsii myagkikh tkaney [Matrix for soft tissue regeneration]. RU patent 2526182. 2013.
- Pashtaev N.P., Pivovarov N.N., Pashtaev A.N., Surkova E.N., Treushnikov V.M., Starostina O.V. Elastichnaya intraokulyarnaya linza [Flexible intraocular lens]. RU patent 2485916. 2011.
- Gushchina M.B., Treushnikov V.V. Implantat orbital’nyy [Orbital implant]. RU patent 2504348. 2011.
- Abelevich A.I., Ovchinnikov E.A., Treushnikov V.M., Treushnikov V.V., Sorokina O.V. Endoprotez dlya lecheniya parakolostomicheskikh gryzh [Endoprosthesis for paracolostomal hernia treatment]. RU patent 2503430. 2012.
- Khomutinnikova N.E., Durnovo E.A., Treushnikov V.M., Treushnikov V.V., Sorokina O.V. Implantat dlya plastiki posttravmaticheskikh defektov i deformatsiy dna i stenok glaznitsy [Implant for plastic repair of posttraumatic defects and orbital fundus and wall deformities]. RU patent 2487726. 2011.










