Modern Techniques for Stem Cells in vivo Imaging (Review)
Application of existing techniques and development of novel approaches to in vivo imaging of particular cell groups for the tasks of cell regenerative medicine is one of the perspective directions in modern biomedical studies. In vivo bioimaging is traditionally employed to study migration direction, proliferation and differentiation of stem cells in experiment and in clinical environment. Currently, numerous techniques for in vivo imaging of cells and cell structures with wide choice of sensitivity, specificity, and resolution characteristics are developed allowing to select an optimal tool for the particular problem. The variety of the modalities provides the opportunity to perform anatomical, physiological pharmaceutical and molecular studies as well as their combinations. Recently the in vivo imaging systems are being continuously updated, the sensitivity of setups increases, and new molecular labels as well as labeling technologies are being developed.
The present review gives an overview of the basic methods for stem cells monitoring and labeling with the discussion of their possibilities, advantages and disadvantages in experimental and clinical studies.
The following classes of techniques for in vivo imaging of cell migration are considered: optical methods (including bioluminescence, fluorescence and optical coherence tomography), non-optical methods (including magnetic resonance imaging and radionuclide imaging), hybrid methods (including optoacoustic tomography) and techniques of multimodal imaging.
Physical characteristics of the outlined methods are analyzed, such as sensitivity, spatial resolution, specificity, maximum imaging depth. Examples of implementation of different techniques for in vivo imaging of migration of stem cells of various origin are given. Basic types of contrasting agents used to enhance contrast, sensitivity and specificity of the discussed imaging modalities are described.
Application of cell technologies in clinical practice grows rapidly. Nowadays stem cells (SC) are employed in regenerative medicine as an advanced treatment of Alzheimer’s and Parkinson’s disease, myocardial infarction, leukemia, diabetes and others [1, 2]. An increased interest toward this class of cells is explained by their unique characteristics such as high proliferative activity, ability for self-renewal, high differentiation capacity.
Cell therapy with SC is conducted in two main directions: local transplantation of cell material and systemic transplantation. In both cases SC are able to migrate, proliferate and repopulate in the pathological focus, showing a therapeutic effect. Note that the efficiency of the cells impact depends not only on their ability to repair damaged tissue, but mostly on their ability to migrate to tissues and organs.
Hence the studies of transplanted cells migration are of high fundamental and applied significance due to the fact that regeneration efficiency in tissues and organs principally depends on the direction and intensity of the process.
As an example of fundamental study one can consider the problem of the risk of tumors after SC transplantation, mechanisms of influence of transplanted SC on tumor development, epigenetic mechanisms of differentiation of SC [3–5]. Investigation of SC mobility in order to determine population viability of transplanted cells for an extended time is an example of applied study [6–8].
Recent methods of SC migration imaging should meet the following requirements: biocompatibility, biosafety, lack of toxicity; the absence of SC genetic modification; the possibility to image a single cell in any anatomical location; quantification of cell migration; reduced imaging during cell division; the presence of contrasting agent only in SC; noninvasiveness of imaging in terms of cell damage over time; stability of cell label during cell life cycle [9].
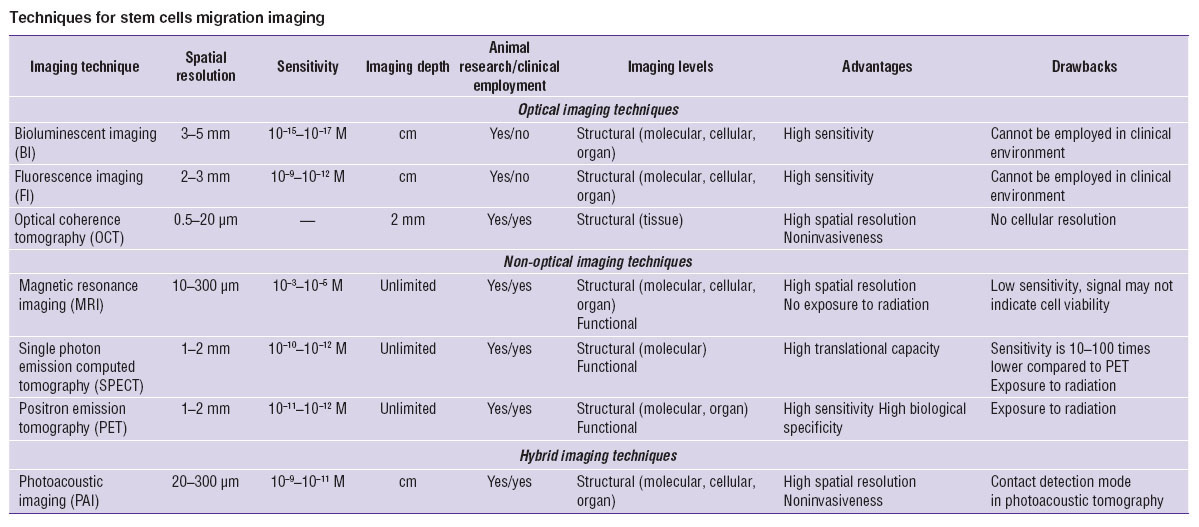
Imaging techniques of in vivo SC visualization are based on different physical principles. They include optical, non-optical and hybrid techniques as well as methods of multimodal imaging [10–13]. Main classes of the above mentioned techniques are summarized in the Table. Most of the presented technologies are realized with the implementation of contrast-increasing agents both of exogenous and endogenous origin which allows to improve contrast, sensitivity and specificity of imaging.
 Techniques for stem cells migration imaging Techniques for stem cells migration imaging
|
The present review is aimed to analyze modern techniques of SC monitoring and labeling with the discussion of their possibilities, advantages and disadvantages in experimental and clinical studies.
Optical imaging techniques
Methods of optical bioimaging are based on principles of collecting light signal emitted by a biological object after specific illumination followed by instrumental processing and numerical analysis of the collected signal in order to achieve 3D images of the object’s structure with high spatial resolution.
Optical imaging techniques include, in particular, fluorescence and bioluminescence imaging which can be divided by into microscopy methods with cellular resolution (microscopy techniques), imaging techniques with spatial resolution at tissue level and methods with resolution at the level of the whole organism.
As most cell structures are achromatic, special approaches for contrasting are used (mostly in fluorescence techniques) to form a structured image of an object. They are based on implementation of contrast agents (markers) that can be either endogenous fluorophores, normally found in cells, or exogenous markers [14]. The latter are divided into direct nonspecific markers (fluorescent dyes, nanoparticles) and indirect specific markers (reporter genes).
In modern fluorescence imaging (FI) an object is typically illuminated by narrow band visible light to excite fluorescing target molecules, or fluorophores. Fluorophores emit light shifted to longer wavelength which is registered by a detector.
Both types of contrast agents are used in FI. Endogenous markers include amino acids (tryptophan, tyrosine, phenylalanine), structural proteins (collagen, elastin), enzymes in the reduced and oxidized forms (NAD, NADP, FAD), vitamins A, D, K and their components, some of lipids and lipoproteins (phospholipids, lipofuscin), different groups of porphyrins (Copro, Uro, Proto) [15]. The main difficulty of endogenous markers implementation for SC visualization is their limited usefulness for in vivo imaging.
Direct non-specific labeling of cells is performed using exogenous fluorescent dyes that are classified as membrane, cytoplasmic and nuclear markers etc. by the target cellular component they interact with [16].
Membrane fluorescent dyes are lipophilic derivatives of carbocyanines. Investigation of in vivo SC migration is typically performed using dyes from PKH family [17] which are known for long retention within a cell (up to a month). Cytoplasmic fluorescent dyes include Calcein, and fluorescein based dyes BCECF, FDA, CFDA, CFDA-AM, CFSE [18]. Typical nuclear dyes are DAPI and Hoechst 33342 which bind to AT-rich sequence of DNA minor groove [19]. The advantages of these groups of dyes are their cost effectiveness, ease of fabrication, lack of genetic modification of cells and the possibility of applying for proliferation studies. Their disadvantages are dye removal from cells over time, and transfer of dye into surrounding cells.
Exogenous markers also include different types of nanoparticles. The advantages of nanoparticles are unique physical properties, non-invasiveness, reasonable cost, easy accessibility [20, 21]. In optical fluorescence bioimaging one employs fluorescent polymer nanoparticles [22], silica nanoparticles [23] and quantum dots [24].
Indirect specific labeling of SC can be performed using methods of genetic labeling. The selected group of cells is subjected to transfection or viral transduction by reporter constructs that encode a label protein under the control of a specific promoter [25]. The most widely used in FI genetic labels are the family of fluorescent proteins isolated from coelenterates, with its best known representative, green fluorescent protein (GFP) isolated from Pacific jellyfish, Aequoria victoria. Fluorescent proteins, when expressed under the control of regulatory elements of the target protein as part of a reporter construct, enable both in vivo and in vitro optical imaging of the target protein expression [26].
The simplest modality of in vivo FI at organism level is surface imaging which enables fast evaluation (1–2 s) of the size of fluorescence area located close to the object’s surface. Employment of high sensitive detectors allows to reveal fluorescence from large depths, yielding however significant image blur due to strong scattering of light within biotissues. In recent years systems for surface FI became commercially available (IVIS, Maestro, Kodak; USA). These imaging systems implement epiluminescence, transluminescence and 3D fluorescence diffuse tomography modalities [27].
The FI techniques are widely used when studying the role of SC in carcinogenesis for understanding of biodistribution, tropism and interaction of SC with tumors. Hereafter, we will concentrate on examples of problems connected with examination the of the SC role in carcinogenesis, as they demonstrate the abilities of in vivo imaging techniques and are extremely important to expand the therapeutic use of SC.
In vivo FI allowed to study the distribution of GFP labeled allogenic SC in immunodeficient mice [28]. Wolf et al. [29] observed migration of systemically administered labeled human SC to lung metastases induced by injection of mouse renal carcinoma cells.
The FI and laser scanning microscopy techniques were employed to study the interaction of tumor with mesenchymal SC in cervical cancer model [30]. Adipose stromal cells labeled with red fluorescent protein Turbo FP635 were shown to migrate into recipient spleen when administered systemically, and into the bone marrow, lung and tumor tissue when administered both systemically and locally.
Bioluminescent imaging (BI). In this method the core genetic label in the reporter construct is luciferase which is a protein that catalyzes reactions accompanied by the emission of light, or bioluminescence [31]. The most common enzyme for in vivo BI is firefly luciferase isolated from the firefly Photinus pyralis. Luciferase oxidizes its substrate luciferin in the presence of oxygen and ATP, and this reaction is accompanied by broadband light emission with the maximum at the wavelength λ=560 nm. Luciferase can serve as a marker of different molecules expression in individual cells or in laboratory animals in vivo. Bioluminescent tomography systems are used to monitor expression of target molecules labeled with luciferase [32].
Luciferase reporter systems allow for in vitro study of gene expression, activity of cellular receptors, signal transmission paths, RNA processing and protein-protein interactions [33]. BI is also employed for characterization and quantification of specific proteins expression after stimulation [34], as well as in the study of the apoptotic process [35]. This imaging modality benefits from the use of an enzyme which is highly specific for its substrate, the absence of background radiation in living tissues, and high sensitivity (~10–15–10–17 М) [36].
BI is used for SC imaging in various biomedical applications such as study of hematopoietic SC migration [37], migration and proliferation of transplanted SC in models of brain disease [38], participation of SC in vessel regeneration [39], differentiation into insulin-producing cells in diabetes [40], assessment of viability of the cardiomyocytes derived from induced pluripotent cells in the infarcted area in myocardial infarction [41].
For example, employment of BI allowed to study the possibility of the use of mesenchymal precursor cells as vectors for gene therapy of tumors [42]. The authors demonstrated attraction of mesenchymal precursor cells carrying anti-tumor agents to tumors.
This approach was also used to study migration and proliferation of SC in tumor and demonstrated attraction of Firefly luciferase labeled SC to microenvironment of irradiated tumors [43]. Kidd et al. [44] demonstrated migration of systemically administered SC in the human lung, liver and spleen of immunodeficient SCID mice and selective accumulation of SC in developing tumors.
BI is also employed to study the effect of SC on tumor growth. The progress in tumor growth at simultaneous injection of luciferase labeled human adenocarcinoma tumor cells (SKOV-3) and human SC was demonstrated in vivo. [45]. On the other hand, BI demonstrated SC inhibitory effect on tumor growth, irrespective of tumor model and way of SC administration [46].
The area of BI applications continues to grow due to technical solutions (for example, high sensitive CCD cameras) allowing to increase the stability of the registered signal, and to design and commercialize diverse modified luciferase forms and corresponding substartes with optimized properties [47].
Direct comparison between bioluminescence and fluorescence imaging techniques for in vivo has not been done yet. However, BI techniques benefit from lower background signal level. The advantage of FI consists in ability to study fixed tissues and perform other modalities of fluorescence analysis (cytometry and cell sorting) in addition to in vivo studies. Employment of FI does not require an expensive substrate, and there exists much wider variety of labels for FI compared to BI.
Another class of bioimaging techniques is based on various effects of light scattering in biological tissues. They include, in particular, optical coherence tomography (OCT).
OCT is noninvasive technique for imaging internal structure of biotissues with the spatial resolution at cell level which is based on interferometric detection of backscattered light of near infrared optical range. This technique is characterized by high spatial resolution, non-invasiveness, and allows for in vivo imaging of 3D biotissue structure in real time with the imaging depth of up to 2 mm [48].
Employment of OCT for SC imaging requires adaptation of the method as well as application of specific contrasting agents, e.g. magnetic micro- and nanoparticles. For example, magnetomotive OCT has been developed to visualize SC labeled with magnetic micro- and nanoparticles on agar scaffolds by analyzing speckle structure of 3D OCT images [49]. OCT was used to image stem-cell-derived photoreceptor precursor cells transplanted into rats with retinal dystrophy. Together with fluorescence confocal scanning laser ophthalmoscope OCT technique allowed for visualization of cell viability up to 15 days after transplantation [50].
In a rat model for neural stem cell (NSC) transplantation studies in which NSCs were modified with brain-derived neurotrophic factor genes, survival, migration, and differentiation of donor cells in host retinas were observed with OCT, Heidelberg retina angiograph, and immunohistochemistry, respectively [51].
The main disadvantage of OCT for SC studies is its poor resolution at cellular level which limits the possibilities of OCT as a method for optical biopsy [48]. Researchers’ efforts are aimed at increasing the resolution of OCT and minimizing the limitations of this technique. In this respect one employs novel contrast agents (magnetic micro- and nanoparticles), optical clearing approach to increase imaging depth and the image contrast, broadband light sources (femtosecond lasers) to obtain images of biological tissues with a resolution of up to several micrometers [48]. Note that OCT is mainly used as a complementary method to visualize the structure of the tissue in which transplanted SC migrate, and not as the primary imaging technique.
Non-optical imaging techniques
Non-optical imaging includes a wide range of techniques based on different physical principles of signal detection, however, for SC monitoring primarily magnetic resonance imaging and radionuclide techniques are employed. Non-optical imaging provides high spatial resolution at relatively large imaging depth, has significant sensitivity and provides information about structural (organ, tissue, cellular, molecular) and functional components. Moreover, non-optical techniques allow to reveal SC with exiting contrast and resolution not only in experimental conditions, but also in clinical environment.
Magnetic resonance imaging (MRI) is based on the detection of signal from hydrogen nuclei within the structure of various compounds of living organism, primarily water molecules. Typically, the contrast based on water content is used only to create anatomical images in which different tissues are distinguished by amount of water.
In modern studies additional contrast agents for MRI are implemented. Gadolinium-based compounds are most effective contrast agents because of unpaired electrons [52, 53]. Chelated gadolinium complexes (Gd3+) are used for SC labeling [54, 55]. Gd3+ containing particles and macromolecules are used as a new generation of contrast agents [56, 57]. The main disadvantage of methods using paramagnetic agents is that they are not sensitive enough to visualize single cells [58].
Another type of contrast agents for MRI is superparamagnetic iron oxide particles (SPIOs) [59]. These particles consist of iron oxide cores, which usually contain a few thousand atoms of iron, which increases local iron concentration and allow to detect lower cell concentrations [60]. Superparamagnetic iron oxide nanoparticles (SPIONs) are the most preferred contrast agents for SC monitoring due to their high sensitivity and excellent biocompatibility [61, 62].
Numerous studies are performed with a novel class of contrast agents based on saturation transfer due to chemical exchange (CEST) [63]. These agents provide additional features such as the ability to distinguish several biological events at one image or simultaneously monitor various cell populations using multiple contrast agents [64]. PARACEST-agents, in which transfer of saturation magnetization is carried out from nuclei of coordinated water (water near the paramagnetic center) to nuclei of free water, are of particular interest [65].
A detailed review by Kircher et al. [66] summarizes different contrast agents used in MRI and considers various possibilities of their application for cell imaging.
For cell labeling the following approaches are employed: 1) direct labeling with magnetic nanoparticles when contrast agents penetrate into cells due to transfection polycationic agents, liposomes, “gene gun”, microinjection, electroporation, or receptor-mediated endocytosis; 2) stable transduction by reporter genes encoding specific proteins, such as intracellular metalloproteinase (transferrin and ferritin) producing signal due to internal iron accumulation.
High sensitivity, resolution, specificity, and the ability to perform the study in a dynamic mode make MRI an attractive technique for SC biology study [67].
In paper [68] porcine SC were transduced by human ferritin. In the in vivo model of porcine myocardial infarction transplanted SC were detected by MRI after 4 weeks. It is shown that ferritin had no effect on reparative or differentiation potential of SC. In certain cases, this labeling method is preferable rather than direct labeling, since it depends on the gene expression which correlates with cell viability and provides more functional information.
Studies on human SC applications demonstrated that SC labeled with SPIOs retain the ability for survival, migration and integration after in vivo transplantation which allows to track the fate of labeled cells in different conditions of administration over time [69], and in various tumor models in mice, rats, rabbits and pigs [70]. In human breast cancer model MRI showed homing of human SPOIs-labeled SC to lung metastases [71]. Tracing of small populations of labeled neural SC in experimental model of stroke is demonstrated [72]. The sensitivity of MRI was sufficient to detect 1,000 labeled SC at co-injection with breast cancer cells in a model of subcutaneous tumors [73]. In the paper by Watada et al. [74] MRI monitoring of SPIOs-labeled SC transplanted into ear and cochlea after 4 weeks after transplantation was performed.
Compounds based on gadolinium chelates are widely applied to study migration and differentiation of SC of various origins and in various models. One of the commonly used materials is a complex of gadolinium with diethylenetriaminepentaacetic acid (Gd-DTPA). Paper [75] shows the possibility of its application for in vivo monitoring labeled mesenchymal SC due to high signal intensity and lack of negative effect on cell viability and proliferation. In [76] the authors used three Gd-DOTA-peptide complexes (1, 4, 7, 10-tetraazacyclododecane-N, N’, N’’, N’-tetraacetic acid) as a contrast agent for labeling mesenchymal SC.
In review [77] unique opportunities for CEST-contrast agents are considered, their physical properties and key features are described; in [78] techniques and tools for MRI screening of CEST agents are formulated. The authors of [79] demonstrated possibilities of CEST to visualize dynamic changes in cell-free hydrogel components in vivo. Composite hydrogels are used in regenerative medicine as tissue-mimicking scaffolds for improving stem cell survival.
The disadvantage of MRI is the presence of artifacts in the images. To overcome this problem a special agent based on nanoparticles for dual-mode filtering of artifacts MRI images (AFIA) is developed, which comprises a combination of the paramagnetic and superparamagnetic nano-material [80]. Using AFIA the artifacts in raw image were eliminated, which enhanced the accuracy of MRI. The authors demonstrated AFIA ability for in vitro samples and a possibility for artifact-free imaging of SC migration in vivo.
Radionuclide imaging (RI) is based on the ability of special detection hardware to monitor the distribution of radiolabelled bioactive compounds in the body which allows to study such processes as metabolism, substance transport, the ligand-receptor interaction, expression of genes. This method has very high sensitivity (<10–9 M) and is able to image biologically active compounds at extremely low concentrations.
Two basic strategies are employed for contrasting in RI: direct labeling with a radioactive marker and labeling by reporter genes.
RI uses two tomographic techniques: single photon emission computed tomography and positron emission tomography [81].
Single photon emission computed tomography (SPECT) is based on registration of radioactivity with a gamma-camera rotating around the studied object at different angles allowing for section image reconstruction. SPECT provides volumetric images of the distribution of radionuclides belonging to pure gamma emitters: technetium-99m (99mTc), indium-111 (111In), iodine-123 (123I), iodine-131 (131I), as well as short lived and ultra-short-lived isotopes: oxygen-15 (15O), carbon-11 (11C), nitrogen-13 (13N), and fluorine-18 (18F) [82].
The use of radiopharmaceuticals (RP) containing radionuclides is a perspective approach. RP are accumulated only in specific organs and structures while this accumulation may be governed by metabolic processes in tissues or local organ perfusion. RP are fabricated through radiochemical synthesis: the radionuclide is “embedded” in a chemical. The fabrication requires radionuclide preparation and evaluation of its half-life [83].
SPECT is often employed for leukocyte migration monitoring [84], however, SC migration can be monitored as well.
Positron emission tomography (PET) is a radioisotope diagnostics technique. It is based on detection of positrons emitted by radionuclides. Positron emitting radioisotopes produce high-energy gamma rays able to penetrate deep into the tissue, resulting in the possibility of applying this approach for studies either in small animals or in human subjects [85]. RP based on technetium-99m (for instance, 99mTc-hexamethylpropyleneamineoxime with half-life of about 6 h which is employed for short term imaging of SC in vivo) provide imaging with high contrast [86].
PET also employs reporter genes technologies. Such genes are divided into three different classes: encoding genes (receptors), enzymes and transporters. When using receptor-based reporters a radiolabel is associated with dopamine receptor, which encodes the reporter gene. Enzyme-based systems use reporter genes to produce specific enzymes that modify radiolabel by inhibiting its release from a cell. The third class of reporter genes is encoded by radiolabel transport proteins (symporters) [87]. Reporter genes systems allow for long term evaluation of injected cells distribution in real time. The advantage of this method is an additional opportunity to study functional activity and cell viability. Currently, one of the most common reporter genes for PET is the herpes simplex virus thymidine kinase of the first type (HSV1-tk) and its mutant (HSV1-sr39tk). This enzyme phosphorylates pyrimidine and adenine bases and is successfully used in combination with radiolabeled reporter probes such as 124I-2-fluoro-2-deoxy-1-D-arabinofuranosyl-5-iodouracil (FIAU), 18F-2-fluoro-2-deoxy-1-D-arabinofuranosyl-5-ethyluracil (FEAU) and 9-(4-18F-fluoro-3-hydroxymethyl-butyl) guanine (18F-FHBG) [88, 89].
Using PET, migration of SC to subcutaneously grafted bowel adenocarcinoma and their proliferation was studied [90]. Monitoring of copper and cobalt isotope-labeled rat SC lasted for more than a month.
Non-optical techniques for SC migration study are widely used in scientific research and clinical trials, however the range of their use depends on the specific problem. MRI has no limitations in the volume and size of the biological object being studied, therefore it perfectly suits for the study of the entire body. At the same time the spatial resolution of MRI varies from 10 to 300 microns depending on system configuration and given the average cell size of 5–50 microns, it may be efficiently employed to study migration of even single transplanted cells. The advantages of MRI include persistence of labels. However, it is difficult to assess the condition of the initially injected cells with this technique. In addition, preservation of the label can be treated as a disadvantage, because the resident macrophages may consume the dead transplanted cells resulting in false positive signal.
SPECT and PET allow for quantitative evaluation of cells and have low background signal, however, compared to MRI and optical imaging techniques, they have a number of disadvantages: low spatial resolution (does not allow for accurate localization of transplanted cells in the organ); radiation load; radioisotope short half-life which limits the duration of cells tracking; nonspecific absorption of radionuclides in normal tissues of internal organs (kidney, liver).
The definite advantage of PET consists in higher sensitivity compared to that of SPECT, which allows for much more accurate evaluation of the number of labeled SC.
Hybrid imaging techniques
Hybrid imaging techniques include photoacoustic imaging (PAI), a hybrid technology which enables imaging basing on detection of ultrasound waves generated by thermoelastic expansion of tissues induced by absorption of probing optical radiation [91]. The object of interest is illuminated by a short laser pulse, pulse energy is absorbed within the inner structures of the object leading to fast temperature increase and thermal expansion. The thermal expansion produces ultrasound waves propagating through object which are detected by ultrasonic transducers located at the object surface. Detected signals are processed resulting in distribution map of absorption coefficient at the optical probing wavelength within the object. The origin of contrast in PAI consists in difference in local optical absorption coefficient determined by endogenous and exogenous chromophores [92].
PAI techniques include two basic modalities: photoacoustic tomography (PAT) and photoacoustic microscopy (PAM) [93].
PAT is based on detection of acoustic signal on the surface of the object irradiated by a pulsed laser [94]. It enables 3D imaging in real time with imaging depth up to several centimeters [95], however, it cannot provide resolution at cellular level. For detection of ultrasound waves PAT requires contact detection mode due to pronounced decay of ultrasound waves in the air between the object and ultrasound transducer. This fact significantly limits application of PAT in biomedical studies [96].
High resolution in PAM is based on tight optical focusing of the probing radiation. A separate direction, optical resolution photoacoustic microscopy (ORPAM) can be distinguished in PAM [97], which can provide spatial resolution of units of microns at depths up to 1 mm in brain [98]. ORPAM provides imaging based on specific optical absorption bands of particular cellular components, chromophores, such as DNA, RNA, cytochromes, myelin, melanin, and hemoglobin [99]. It enables determination of cell viability, state of the cell membrane components and, if possible, evaluation of cellular metabolism through the ratio of reduced and oxidized cytochromes forms [100].
Contrasting agents in PAI belong to both endogenous and exogenous chromophores. Different exogenous contrasting agents employed in PAI can be separated into five basic groups: molecular fluorescent dyes (ICG, Alexa Fluor 750, BHQ3, QXL680, IRDye 800CW, MMPSense™), noble metal plasmon resonance particles (gold and silver nanoparticles), other types of nanoparticles (quantum dots, fluorescent silica nanoparticles), multimodal contrast agents (gadolinium chelate complexes, single-layer carbon nanotubes) and contrast agents for theranostics (SPIOs, gold and silica nanoparticles) [101].
PAI technique is widely used in different areas of cell therapy. A number of papers study regenerative functions of SC in pathological foci of different origin. For example, Ricles et al. [102] employed in vitro labeling of mesenchymal rat SC and macrophages by gold nanoparticles. Using a model of lower limb ischemia in rats they also showed the ability to monitor labeled SC in vivo and to quantify macrophage infiltration lesions by PAI followed by histological techniques and mass spectrometry to verify the results.
Noninvasive monitoring of SC used to accelerate the process of healing of superficial skin injuries (burns, trophic ulcers, etc.) is of high interest in experimental and clinical studies. An example of this trend is the paper [103], where structural damage of tissue and blood supply to the epidermis and dermis in skin burn model in rats were evaluated by PAI before and after implantation of 3D fibrin gel scaffolds with adipose tissue SC labeled by gold nanoparticles.
A novel, rapidly developing modification of PAI is photoacoustic flow cytometry (PAFC) which is aimed at detection of cells labeled by exogenous contrast agents in a stream of physiological fluids (blood, lymph, cerebrospinal fluid) [104]. Using this technique, the presence of markers of leukemia, carcinomas, melanomas, and cancer stem cells can be detected in preclinical animal models [105].
Thus, we demonstrated that PAI has strong potential for wide application in cell therapy: from monitoring in SC therapy and tissue engineering at the molecular level to detection of individual cells in the fluid flow at the level of the entire organism due to its noninvasiveness, safety, selectivity and capacity to provide long-term monitoring of different processes.
Multimodal imaging techniques
Multimodal imaging combines two or more imaging techniques to study the same object and produce integrated data (at several different levels) which can complement each other and provide complete information about the object. Commonly, techniques from different groups are combined, for example, optical techniques are combined with non-optical ones, however, combinations within the same group are also possible.
Combination of optical and non-optical techniques is commonly used to study migration of SC in the recipient organism because it provides both high spatial resolution and high sensitivity, and accurately reveals localization of SC.
Cao et al. [106] successfully evaluated viability, proliferation and migration after intracardiac administration of mouse embryonic SC transfected simultaneously with three reporter genes (RFP, Luciferase, and HSV1-tk) using BI and PET techniques.
The authors of paper [107] used multimodal imaging technology and transfection of various reporter genes to monitor transplanted neural progenitor cells (NPCs) and their functional status. Using BI and PET techniques, they investigated NPCs expressing HSV1-tk, GFP and firefly luciferase (Fluc) genes in culture and in glioma models in vivo.
Tumor SC were detected in subcutaneous or orthotopically grafted gliomas using fluorescence molecular tomography and PET in presence of antibody-based tracers [108].
Using MRI and BI a comprehensive assessment of transplanted mouse embryonic SC carrying a double label (superparamagnetic iron oxide and luciferase) was performed in a model of myocardial infarction. Exact anatomical localization of SC in the area of infarction was determined by MRI, while viability of SC administrated into myocardial infarction zone was evaluated by BI [109].
MRI and BI were successfully combined to monitor bone marrow mesenchymal SC labeled by reporter genes (Firefly Luciferase and GFP) and iron oxide particles transplanted into CNS of immunocompetent mice [110].
Combination of non-optical techniques allows for simultaneous study of viability and localization of SC.
A combination of SPECT and MRI techniques allows observing tracking of intraperitoneally administered mesenchymal SC of human bone marrow in mice with neuroblastoma and detection of accumulation of mesenchymal SC labeled by 111In-oxine in tumors, 48 h after transplantation [111].
In paper [112] marrow SC labeled by iron oxide and implanted into the rat striatum in Parkinson’s disease model were visualized using MRI. MRI demonstrated the presence of labeled mesenchymal SC in the affected area up to 28 days after transplantation. Functional activity of exogenously injected cells labeled by radiopharmaceutical was effectively evaluated by PET.
Combination of optical techniques allows for high sensitive simultaneous study of time evolution of SC small populations distribution, colocalization and interaction of SC with microenvironment in various models of pathological process.
FI and BI were successfully employed for study of distribution and differentiation of SC in mice with tumors [113]. The authors used tumor and stem cells labeled with various reporter genes (fluorescent and bioluminescent).
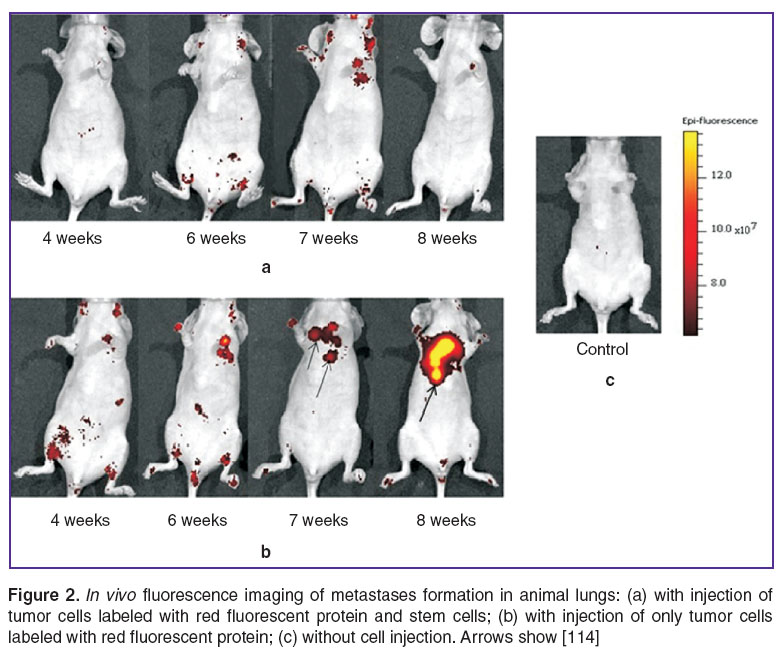
In paper [114] FI and BI techniques were employed to demonstrate the effect of human bone marrow mesenchymal SC on formation of metastases in immunodeficient mice for breast cancer model. SC labeled with luciferase gene and tumor cells carrying red fluorescent protein were used. BI in vivo revealed distribution of SC in lungs and abdominal organs at weeks 2 and 3 after transplantation and subsequent remigration of SC into the lungs at weeks 6and 7 (Figure 1). FI allowed to detect inhibitory effect of SC on formation of lung metastasis (Figure 2).
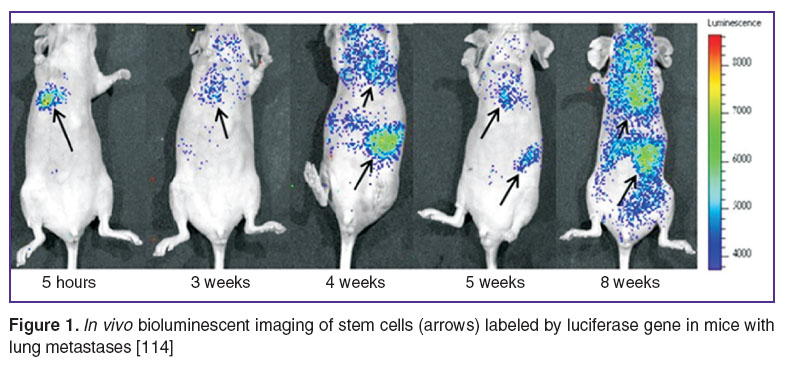 Figure 1. In vivo bioluminescent imaging of stem cells (arrows) labeled by luciferase gene in mice with lung metastases [114] Figure 1. In vivo bioluminescent imaging of stem cells (arrows) labeled by luciferase gene in mice with lung metastases [114]
|
Such multimodal approach to collection of data about location and status of SC can minimize potential drawbacks of particular method employment, and provide suitable information for clinical and research applications.
Conclusion
Rapid progress in development of markers along with improvement of in vivo imaging systems allows for reliable study of direction and efficiency of SC migration. The studies of SC migration in research and in clinical environment have significant differences. The main criteria in the clinical setting are biocompatibility, safety, non-toxicity, and non-invasiveness. Accordingly, the main criteria for the research works studying SC migration are precision, quantitative characterization and long-term monitoring cell migration, as well as label stability [115].
Currently, techniques for imaging cells and their structures in vivo are developed satisfying the requirements of both basic and clinical research. A wide range of such characteristics as sensitivity, resolution, imaging depth, and specificity allows one to choose the optimum experimental technique and conditions (accounting for particular experimental model). Diverse approaches provide an opportunity to perform anatomical, physiological, pharmacological and molecular studies, and in some cases, techniques can be efficiently combined. Currently the systems for in vivo imaging are being actively improved: instruments with higher sensitive are developed along with novel molecular labels, and advanced strategies for cell labeling.
Acknowledgements. The major part of the work was supported by the Russian Scientific Foundation (project No.14-15-00536); the review and comparison of bioluminescent imaging techniques along with bioluminescent contrast agents was supported by the Ministry of Education and Science of Russian Federation in the framework of the governmental task in scientific research (basic part) No.2014/134 (project No.2460).
Conflicts of Interest. The authors have no conflicts of interest.
References
- Acton P.D., Zhou R. Imaging reporter genes for cell tracking with PET and SPECT. Q J Nucl Med Mol Imaging 2005; 49(4): 349–360.
- Chiu R.C. Bone-marrow stem cells as a source for cell therapy. Heart Fail Rev 2003; 8(3): 247–251.
- Li Z., Wu J.C., Sheikh A.Y., Kraft D., Cao F., Xie X., Patel M., Gambhir S.S., Robbins R.C., Cooke J.P., Wu J.C. Differentiation, survival, and function of embryonic stem cell derived endothelial cells for ischemic heart disease. Circulation 2007; 116(11 Suppl): I–46–I–54, http://dx.doi.org/10.1161/CIRCULATIONAHA.106.680561.
- Cao F., Lin S., Xie X., Ray P., Patel M., Zhang X., Drukker M., Dylla S.J., Connolly A.J., Chen X., Weissman I.L., Gambhir S.S., Wu J.C. In vivo visualization of embryonic stem cell survival, proliferation, and migration after cardiac delivery. Circulation 2006; 113(7): 1005–1014, http://dx.doi.org/10.1161/circulationaha.105.588954.
- van der Bogt K.E.A., Swijnenburg R.J., Cao F., Wu J.C. Molecular imaging of human embryonic stem cells: keeping an eye on differentiation, tumorigenicity and immunogenicity. Cell Cycle 2006; 5(23): 2748–2752, http://dx.doi.org/10.4161/cc.5.23.3533.
- Chang G.Y., Xie X., Wu J.C. Overview of stem cells and imaging modalities for cardiovascular diseases. J Nucl Cardiol 2006; 13(4): 554–569, http://dx.doi.org/10.1016/j.nuclcard.2006.05.012.
- Kim D.E., Schellingerhout D., Ishii K., Shah K., Weissleder R. Imaging of stem cell recruitment to ischemic infarcts in a murine model. Stroke 2004; 35(4): 952–957, http://dx.doi.org/10.1161/01.str.0000120308.21946.5d.
- Zhao C., Tian M., Zhang H. In vivo stem cell imaging. Open Nucl Med J 2010; 2: 171–177, http://dx.doi.org/10.2174/1876388X01002010171.
- Frangioni J.V., Hajjar R.J. In vivo tracking of stem cells for clinical trials in cardiovascular disease. Circulation 2004; 110(21): 3378–3383, http://dx.doi.org/10.1161/01.cir.0000149840.46523.fc.
- Tong L., Zhao H., He Z., Li Z. Current perspectives on molecular imaging for tracking stem cell therapy. In: Medical imaging in clinical practice. Okechukwu F.E. (editor). InTech; 2013; р. 73–79, http://dx.doi.org/10.5772/53028.
- Chen Z.-Y., Wang Y.-X., Yang F., Lin Y., Zhou Q.-L., Liao Y.-Y. New researches and application progress of commonly used optical molecular imaging technology. Biomed Res Int 2014, 2014: 429198, http://dx.doi.org/10.1155/2014/429198.
- Gao Y., Cui Y., Chan J., Xu C. Stem cell tracking with optically active nanoparticles. Am J Nucl Med Mol Imaging 2013; 3(3): 232–246.
- Reagan M.R., Kaplan D.L. Concise review: mesenchymal stem cell tumor-homing: detection methods in disease model systems. Stem Сells 2011; 29(6): 920–927, http://dx.doi.org/10.1002/stem.645.
- Solovieva A.O., Zubareva K.E., Poveschenko A.F., Nechaeva E.A., Konenkov V.I. Methods of cells labeling for visualization in vivo. Kletochnaya transplantologiya i tkanevaya inzheneriya 2013; VIII(4): 33–38.
- Koltovoy N.A., Kraevoy S.A. Fluorestsentnye metody diagnostiki v meditsine [Fluorescent diagnostics in medicine]. Moscow: Bookvika.ru; 2014; 228 p.
- Parish C.R. Fluorescent dyes for lymphocyte migration and proliferation studies. Immunol Cell Biol 1999; 77(6): 499–508, http://dx.doi.org/10.1046/j.1440-1711.1999.00877.x.
- Li P., Zhang R., Sun H., Chen L., Liu F., Yao C., Du M., Jiang X. PKH26 can transfer to host cells in vitro and vivo. Stem Cells Dev 2013; 22(2): 340–344, http://dx.doi.org/10.1089/scd.2012.0357.
- Weston S.A., Parish C.R. New fluorescent dyes for lymphocyte migration studies: analysis by flow cytometry and fluorescence microscopy. J Immunol Methods 1990; 133(1): 87–97, http://dx.doi.org/10.1016/0022-1759(90)90322-M.
- Leiker M., Suzuki G., Iyer V.S., Canty J.M. Jr., Lee T. Assessment of a nuclear affinity labeling method for tracking implanted mesenchymal stem cells. Cell Transplant 2008; 17(8): 911–922, http://dx.doi.org/10.3727/096368908786576444.
- Wang Y., Xu C., Ow H. Commercial nanoparticles for stem cell labeling and tracking. Theranostics 2013; 3(8): 544–559, http://dx.doi.org/10.7150/thno.5634.
- Гусев А.И. Наноматериалы, наноструктуры, нанотехнологии. М: Физматлит; 2005; 416 с. Gusev A.I. Nanomaterialy, nanostruktury, nanotekhnologii [Nanomaterials, nanostructures, nanotechnology]. Moscow: Fizmatlit; 2005; 416 p.
- Ziganshin A.U., Ziganshina L.E. Nanoparticles: pharmacological expectancies and toxicological problems. Kazanskiy meditsinskiy zhurnal 2008; 89(1): 1–7.
- Burns A., Ow H., Wiesner U. Fluorescent core-shell silica nanoparticles: towards “Lab on a Particle” architectures for nanobiotechnology. Chem Soc Rev 2006; 35(11): 1028–1042, http://dx.doi.org/10.1039/B600562B.
- Rempel' A.A. Quantum dots for technology and medicine. Vestnik Ural’skogo otdeleniya RAN 2010; 32(2): 45–51.
- Rodriguez-Porcel М. In vivo imaging and monitoring of transplanted stem cells: clinical applications. Curr Cardiol Rep 2010; 12(1): 51–58, http://dx.doi.org/10.1007/s11886-009-0073-1.
- Morozova E.S., Verkhusha V.V., Perskiy E.E. Fluorescent proteins of the red spectral region. Visnyk Harkivs’kogo nacional’nogo universytetu im. V.N. Karazina Ser. Biologija 2009; 856(9): 29–38.
- Kuo C., Coquoz O., Troy T.L., Xu H., Rice B.W. Three-dimensional reconstruction of in vivo bioluminescent sources based on multi-spectral imaging. J Biomed Opt 2007; 12(2): 1–12, http://dx.doi.org/10.1117/1.2717898.
- Li Z., Liao W., Cui X., Zhao Q., Liu M., Chen Y., Liu T., Liu N., Wang F., Yi Y., Shao N. Intravenous transplantation of allogeneic bone marrow mesenchymal stem cells and its directional migration to the necrotic femoral head. Int J Med Sci 2011; 8(1): 74–83, http://dx.doi.org/10.7150/ijms.8.74.
- Wolf D., Rumpold H., Koeck R. Re: Mesenchymal stem cells: potential precursors for tumor stroma and targeted-delivery vehicles for anticancer agents. J Natl Cancer Inst 2005; 97(7): 540–542, http://dx.doi.org/10.1093/jnci/dji088.
- Meleshina А.V., Cherkasova Е.I., Sergeeva Е.А., Kleshnin М.S., Turchin I.V., Kiseleva Е.V., Dashinimaev E.V., Shirmanova М.V., Lukyanov S.А., Zagaynova Е.V. The study of the interaction of mesenchymal stem cells and the tumor using the methods of fluorescent bioimaging. Sovremennye tehnologii v medicine 2012; 4: 7–16.
- Konopka R., Hýzdalová M., Kubala L., Pacherník J. New luminescence-based approach to measurement of luciferase gene expression reporter activity and adenosine triphosphate-based determination of cell viability. Folia Biol (Praha) 2010; 56(2): 66–71.
- Kuchmiy A.A., Efimov G.A., Nedospasov S.A. Methods for in vivo molecular imaging. Biochemistry (Moscow) 2012; 77(12): 1339–1353, http://dx.doi.org/10.1134/s0006297912120012.
- Marques S.M., Esteves da Silva J.C.G. Firefly bioluminescence: a mechanistic approach of luciferase catalyzed reactions. IUBMB Life 2009; 61(1): 6–17, http://dx.doi.org/10.1002/iub.134.
- Sato A., Klaunberg B., Tolwani R. In vivo bioluminescence imaging. Comp Med 2004; 54(6): 631–634.
- Hickson J., Ackler S., Klaubert D., Bouska J., Ellis P., Foster K., Oleksijew A., Rodriguez L., Schlessinger S., Wang B., Frost D. Noninvasive molecular imaging of apoptosis in vivo using a modified firefly luciferase substrate, Z-DEVD-aminoluciferin. Cell Death Differ 2010; 17(6): 1003–1010, http://dx.doi.org/10.1038/cdd.2009.205.
- Paroo Z., Bollinger R.A., Braasch D.A., Richer E., Corey D.R., Antich P.P., Mason R.P. Validating bioluminescence imaging as a high-throughput, quantitative modality for assessing tumor burden. Mol Imaging 2004; 3(2): 117–124, http://dx.doi.org/10.1162/1535350041464865.
- Lin Y., Molter J., Lee Z., Gerson S.L. Bioluminescence imaging of hematopoietic stem cell repopulation in murine models. Methods Mol Biol 2008; 430: 295–306, http://dx.doi.org/10.1007/978-1-59745-182-6_20.
- Tennstaedt A., Aswendt M., Adamczak J., Hoehn M. Noninvasive multimodal imaging of stem cell transplants in the brain using bioluminescence imaging and magnetic resonance imaging. Methods Mol Biol 2013; 1052: 153–166, http://dx.doi.org/10.1007/7651_2013_14.
- Huang N.F., Okogbaa J., Babakhanyan A., Cooke J.P. Bioluminescence imaging of stem cell-based therapeutics for vascular regeneration. Theranostics 2012; 2(4): 346–354, http://dx.doi.org/10.7150/thno.3694.
- Lee S., Youn H., Chung T., Hwang do W., Oh S.W., Kang K.W., Chung J.-K., Lee D.S. In vivo bioluminescence imaging of transplanted mesenchymal stem cells as a potential source for pancreatic regeneration. Mol Imaging 2014; 13: 1–12.
- Lepperhof V., Polchynski O., Kruttwig K., Brüggemann C., Neef K., Drey F., Zheng Y., Ackermann J.P., Choi Y.H., Wunderlich T.F., Hoehn M., Hescheler J., Sarić T. Bioluminescent imaging of genetically selected induced pluripotent stem cell-derived cardiomyocytes after transplantation into infarcted heart of syngeneic recipients. PLoS One 2014; 9(9): e107363, http://dx.doi.org/10.1371/journal.pone.0107363.
- Komarova S., Kawakami Y., Stoff-Khalili M.A., Curiel D.T., Pereboeva L. Mesenchymal progenitor cells as cellular vehicles for delivery of oncolytic adenoviruses. Mol Cancer Ther 2006; 5(3): 755–766, http://dx.doi.org/10.1158/1535-7163.mct-05-0334.
- Klopp A.H., Spaeth E.L., Dembinski J.L., Woodward W.A., Munshi A., Meyn R.E., Cox J.D., Andreeff M., Marini F.C. Tumor irradiation increases the recruitment of circulating mesenchymal stem cells into the tumor microenvironment. Cancer Res 2007; 67(24): 11687–11695, http://dx.doi.org/10.1158/0008-5472.CAN-07-1406.
- Kidd S., Spaeth E., Dembinski J.L., Dietrich M., Watson K., Klopp A., Battula V.L., Weil M., Andreeff M., Marini F.C. Direct evidence of mesenchymal stem cell tropism for tumor and wounding microenvironments using in vivo bioluminescent imaging. Stem Cells 2009; 27(10): 2614–2623, http://dx.doi.org/10.1002/stem.187.
- Spaeth E.L., Dembinski J.L., Sasser A.K., Watson K., Klopp A., Hall B., Andreeff M., Marini F. Mesenchymal stem cell transition to tumor-associated fibroblasts contributes to fibrovascular network expansion and tumor progression. PLoS One 2009; 4(4): e4992, http://dx.doi.org/10.1371/journal.pone.0004992.
- Kéramidas M., Fraipont F., Karageorgis A., Moisan A., Persoons V., Richard M.-J., Coll J.-L., Rome C. The dual effect of mesenchymal stem cells on tumour growth and tumour angiogenesis. Stem Cell Res Ther 2013; 4(2): 41, http://dx.doi.org/10.1186/scrt195.
- Miraglia L., King F., Damoiseaux R. Seeing the light: luminescent reporter gene assays. Comb Chem High Throughput Screen 2011; 14(8): 648–657, http://dx.doi.org/10.2174/138620711796504389.
- Rukovodstvo po opticheskoy kogerentnoy tomografii [Handbook of optical coherence tomography]. Pod red. Gladkovoy N.D., Shakhovoy N.M., Sergeeva A.M. [Gladkova N.D., Shakhova N.M., Sergeev A.M. (editors)]. Moscow: Fizmatlit, Medkniga; 2007; 296 p.
- Cimalla P., Werner T., Winkler K., Mueller C., Wicht S., Gaertner M., Mehner M., Walther J., Rellinghaus B., Wittig D., Karl M.O., Ader M., Funk R.H., Koch E. Imaging of nanoparticle-labeled stem cells using magnetomotive optical coherence tomography, laser speckle reflectometry, and light microscopy. J Biomed Opt 2015; 20(3): 036018, http://dx.doi.org/10.1117/1.JBO.20.3.036018.
- Laver C.R.J., Metcalfe A.L., Szczygiel L., Yanai A., Sarunic M.V., Gregory-Evans K. Bimodal in vivo imaging provides early assessment of stem-cell-based photoreceptor engraftment. Eye (Lond) 2015; 29(5): 681–690, http://dx.doi.org/10.1038/eye.2015.24.
- Zhou X., Sun J., Yuan H., Wu D., Zhou X., Sun D., Li H., Shao Z., Zhang Z. A rat model for studying neural stem cell transplantation. Acta Pharmacol Sin 2009; 30(11): 1496–1504, http://dx.doi.org/10.1038/aps.2009.151.
- Liu G., Wang Z., Lu J., Xia C., Gao F., Gong Q., Song B., Zhao X., Shuai X., Chen X., Ai H., Gu Z. Low molecular weight alkyl-polycation wrapped magnetite nanoparticle clusters as MRI probes for stem cell labeling and in vivo imaging. Biomaterials 2011; 32(2): 528–537, http://dx.doi.org/10.1016/j.biomaterials.2010.08.099.
- Rudelius M., Daldrup-Link H.E., Heinzmann U., Piontek G., Settles M., Link T.M., Schlegel J. Highly efficient paramagnetic labelling of embryonic and neuronal stem cells. Eur J Nucl Med Mol Imaging 2003; 30(7): 1038–1044, http://dx.doi.org/10.1007/s00259-002-1110-0.
- Kim K.S., Park W., Na K. Gadolinium-chelate nanoparticle entrapped human mesenchymal stem cell via photochemical internalization for cancer diagnosis. Biomaterials 2015; 36: 90–97, http://dx.doi.org/10.1016/j.biomaterials.2014.09.014.
- Liu Y., He Z.J., Xu B., Wu Q.Z., Liu G., Zhu H., Zhong Q., Deng D.Y., Ai H., Yue Q., Wei Y., Jun S., Zhou G., Gong Q.Y. Evaluation of cell tracking effects for transplanted mesenchymal stem cells with jetPEI/Gd-DTPA complexes in animal models of hemorrhagic spinal cord injury. Brain Res 2011; 1391: 24–35, http://dx.doi.org/10.1016/j.brainres.2011.03.032.
- Lin G., Zhu W., Yang L., Wu J., Lin B., Xu Y., Cheng Z., Xia C., Gong Q., Song B., Ai H. Delivery of siRNA by MRI-visible nanovehicles to overcome drug resistance in MCF-7/ADR human breast cancer cells. Biomaterials 2014; 35(35): 9495–9507, http://dx.doi.org/10.1016/j.biomaterials.2014.07.049.
- Tseng C.L., Shih I.L., Stobinski L., Lin F.H. Gadolinium hexanedione nanoparticles for stem cell labeling and tracking via magnetic resonance imaging. Biomaterials 2010; 31(20): 5427–5435, http://dx.doi.org/10.1016/j.biomaterials.2010.03.049.
- Arbab A.S., Liu W., Frank J.A. Cellular magnetic resonance imaging: current status and future prospects. Expert Rev Med Devices 2006; 3(4): 427–439, http://dx.doi.org/10.1586/17434440.3.4.427.
- Wang Y.X., Hussain S.M., Krestin G.P. Superparamagnetic iron oxide contrast agents: physicochemical characteristics and applications in MR imaging. Eur Radiol 2001; 11(11): 2319–2331, http://dx.doi.org/10.1007/s003300100908.
- Bull E., Madani S.Y., Sheth R., Seifalian A., Green M., Seifalian A.M. Stem cell tracking using iron oxide nanoparticles. Int J Nanomedicine 2014; 9(1): 1641–1653, http://dx.doi.org/10.2147/IJN.S48979.
- Yang C.Y., Tai M.F., Chen S.T., Wang Y.T., Chen Y.F., Hsiao J.K., Wang J.L., Liu H.M. Labeling of human mesenchymal stem cell: сomparison between paramagnetic and superparamagnetic agents. J Appl Phy 2009; 105: 07B314, http://dx.doi.org/10.1063/1.3072821.
- Azzabi F., Rottmar M., Jovaisaite V., Rudin M., Sulser T., Boss A., Eberli D. Viability, differentiation capacity, and detectability of super-paramagnetic iron oxide-labeled muscle precursor cells for MRI. Tissue Eng Part C Methods 2015; 21(2): 182–191, http://dx.doi.org/10.1089/ten.TEC.2014.0110.
- Ward K.M., Aletras A.H., Balaban R.S. A new class of contrast agents for MRI based on proton chemical exchange dependent saturation transfer (CEST). J Magn Reson 2000; 143(1): 79–87, http://dx.doi.org/10.1006/jmre.1999.1956.
- Aime S., Carrera C., Castelli D.D., Crich S.G., Terreno E. Tunable imaging of cells labeled with MRI-PARACEST agents. Angew Chem Int Ed Engl 2005; 44(12): 1813–1815, http://dx.doi.org/10.1002/anie.200462566.
- Zhang S., Merritt M., Woessner D.E., Lenkinski R.E., Sherry A.D. PARACEST agents: modulating MRI contrast via water proton exchange. Acc Chem Res 2003; 36(10): 783–790, http://dx.doi.org/10.1021/ar020228m.
- Kircher M.F., Gambhir S.S., Grimm J. Noninvasive cell-tracking methods. Nat Rev Clin Oncol 2011; 8(11): 677–688, http://dx.doi.org/10.1038/nrclinonc.2011.141.
- Trattnig S., Pinker K., Ba-Ssalamah A., Nöbauer-Huhmann I.M. The optimal use of contrast agents at high field MRI. Eur Radiol 2006; 16(6): 1280–1287, http://dx.doi.org/10.1007/s00330-006-0154-0.
- Reagan M.R., Kaplan D.L. Concise review: Mesenchymal stem cell tumor-homing: detection methods in disease model systems. Stem cells 2011; 29(6): 920–927, http://dx.doi.org/10.1002/stem.645.
- Guzman R., Uchida N., Bliss T.M., He D., Christopherson K., Stellwagen D., Capela A., Greve J., Malenka R.C., Moseley M.E., Palmer T.D., Steinberg G.K. Long-term monitoring of transplanted human neural stem cells in developmental and pathological contexts with MRI. Proc Natl Acad Sci USA 2007; 104(24): 10211–10216, http://dx.doi.org/10.1073/pnas.0608519104.
- Anderson S.A., Glod J., Arbab A.S., Noel M., Ashari P., Fine H.A., Frank J.A. Noninvasive MR imaging of magnetically labeled stem cells to directly identify neovasculature in a glioma model. Blood 2005; 105(1): 420–425, http://dx.doi.org/10.1182/blood-2004-06-2222.
- Loebinger M.R., Kyrtatos P.G., Turmaine M., Price A.N., Pankhurst Q., Lythgoe M.F., Janes S.M. Magnetic resonance imaging of mesenchymal stem cells homing to pulmonary metastases using biocompatible magnetic nanoparticles. Cancer Res 2009; 69(23): 8862–8867, http://dx.doi.org/10.1158/0008-5472.CAN-09-1912.
- Daadi M.M., Li Z., Arac A., Grueter B.A., Sofilos M., Malenka R.C., Wu J.C., Steinberg G.K. Molecular and magnetic resonance imaging of human embryonic stem cell-derived neural stem cell grafts in ischemic rat brain. Mol Ther 2009; 17(7): 1282–1291, http://dx.doi.org/10.1038/mt.2009.104.
- Loebinger M.R., Janes S.M. Stem cells as vectors for antitumour therapy. Thorax 2010; 65(4): 362–369, http://dx.doi.org/10.1136/thx.2009.128025.
- Watada Y., Yamashita D., Toyoda M., Tsuchiya K., Hida N., Tanimoto A., Ogawa K., Kanzaki S., Umezawa A. Magnetic resonance monitoring of superparamagnetic iron oxide (SPIO)-labeled stem cells transplanted into the inner ear. Neurosci Res 2015; 95: 21–26, http://dx.doi.org/10.1016/j.neures.2015.01.010.
- Geng K., Yang Z.X., Huang D., Yi M., Jia Y., Yan G., Cheng X., Wu R. Tracking of mesenchymal stem cells labeled with gadolinium diethylenetriamine pentaacetic acid by 7T magnetic resonance imaging in a model of cerebral ischemia. Mol Med Rep 2015; 11(2): 954–960, http://dx.doi.org/10.3892/mmr.2014.2805.
- Cao L., Li B., Yi P., Zhang H., Dai J., Tan B., Deng Z. The interplay of T1- and T2-relaxation on T1-weighted MRI of hMSCs induced by Gd-DOTA-peptides. Biomaterials 2014; 35(13): 4168–4174, http://dx.doi.org/10.1016/j.biomaterials.2014.01.073.
- McMahon M.T., Chan K.W. Developing MR probes for molecular imaging. Adv Cancer Res 2014; 124: 297–327, http://dx.doi.org/10.1016/B978-0-12-411638-2.00009-4.
- Song X., Chan K.W., McMahon M.T. Screening of CEST MR contrast agents. Methods Mol Biol 2011; 771: 171–187, http://dx.doi.org/10.1007/978-1-61779-219-9_9.
- Liang Y., Bar-Shir A., Song X., Gilad A.A., Walczak P., Bulte J.W. Label-free imaging of gelatin-containing hydrogel scaffolds. Biomaterials 2015; 42: 144–150, http://dx.doi.org/10.1016/j.biomaterials.2014.11.050.
- Shin T.H., Choi J.S., Yun S., Kim I.S., Song H.T., Kim Y., Park K.I., Cheon J. T1 and T2 dual-mode MRI contrast agent for enhancing accuracy by engineered nanomaterials. ACS Nano 2014; 8(4): 3393–3401, http://dx.doi.org/10.1021/nn405977t.
- Shmidt V. Opticheskaya spektroskopiya dlya khimikov i biologov [Optical spectroscopy for chemists and biologists]. Moscow: Tekhnosfera; 2007; 368 p.
- Weaner L.E., Hoerr D.C. Synthesis and application of radioisotopes in pharmaceutical research and development. In: Fundamentals of early clinical drug development: from synthesis design to formulation. Abdel-Magid A.F., Caron S. (editors). New York: Wiley; 2006. p. 189–214, http://dx.doi.org/10.1002/0470043407.ch11.
- Mirshojaei S.F., Ahmadi A., Morales-Avila E., Ortiz-Reynoso M., Reyes-Perez H. Radiolabelled nanoparticles: novel classification of radiopharmaceuticals for molecular imaging of cancer. J Drug Target 2015; Jun 10: 1–11, http://dx.doi.org/10.3109/1061186X.2015.1048516 [Epub ahead of print].
- Hong H., Yang Y., Zhang Y., Cai W. Non-invasive cell tracking in cancer and cancer therapy. Curr Top Med Chem 2010; 10(12): 1237–1248, http://dx.doi.org/10.2174/156802610791384234.
- Patriarca F., Carobolante F., Zamagni E., Montefusco V., Bruno B., Englaro E., Nanni C., Geatti O., Isola M., Sperotto A., Buttignol S., Stocchi R., Corradini P., Cavo M., Fanin R. The role of positron emission tomography with 18F-fluorodeoxyglucose integrated with computed tomography in the evaluation of patients with multiple myeloma undergoing allogeneic stem cell transplantation. Biol Blood Marrow Transplant 2015; 21(6): 1068–1073, http://dx.doi.org/10.1016/j.bbmt.2015.03.001.
- Detante O., Moisan A., Dimastromatteo J., Richard M.J., Riou L., Grillon E., Barbier E., Desruet M.D., De Fraipont F., Segebarth C., Jaillard A., Hommel M., Ghezzi C., Remy C. Intravenous administration of 99mTc-HMPAO-labeled human mesenchymal stem cells after stroke: in vivo imaging and biodistribution. Cell Transplant 2009; 18(12): 1369–1379, http://dx.doi.org/10.3727/096368909X474230.
- Kraitchman D.L., Bulte J.W. In vivo imaging of stem cells and beta cells using direct cell labeling and reporter gene methods. Arterioscler Thromb Vasc Biol 2009; 29: 1025–1030, http://dx.doi.org/10.1161/ATVBAHA.108.165571.
- Tjuvajev J.G., Doubrovin M., Akhurst T., Cai S., Balatoni J., Alauddin M.M., Finn R., Bornmann W., Thaler H., Conti P.S., Blasberg R.G. Comparison of radiolabeled nucleoside probes (FIAU, FHBG, and FHPG) for PET imaging of HSV1-tk gene expression. J Nucl Med 2002; 43(8): 1072–1083.
- Kang K.W., Min J.J., Chen X., Gambhir S.S. Comparison of [14C]FMAU, [3H]FEAU, [14C]FIAU, and [3H]PCV for monitoring reporter gene expression of wild type and mutant herpes simplex virus type 1 thymidine kinase in cell culture. Mol Imaging Biol 2005; 7(4): 296–303, http://dx.doi.org/10.1007/s11307-005-0010-7.
- Mirpour S., Gholamrezanezhad A. Clinical stem cell imaging and in vivo tracking. In: Stem cells in clinic and research. Gholamrezanezhad А. (editor). InTech; 2011; р. 637–656, http://dx.doi.org/10.5772/17821.
- Yao J., Xia J., Wang L.V. Multiscale functional and molecular photoacoustic tomography. Ultrason Imaging 2015, http://dx.doi.org/10.1177/0161734615584312 [Epub ahead of print].
- Wang L.V. Multiscale photoacoustic microscopy and computed tomography. Nat Photonics 2009; 3(9): 503–509, http://dx.doi.org/10.1038/nphoton.2009.157.
- Liu J., Tang Z., Wu Y., Wang Y. Rapid and noncontact photoacoustic tomography imaging system using an interferometer with high-speed phase modulation technique. Rev Sci Instrum 2015; 86(4): 044904, http://dx.doi.org/10.1063/1.4918801.
- Xu M.H., Wang L.V. Photoacoustic imaging in biomedicine. Rev Sci Instrum 2006; 77(4): 041101, http://dx.doi.org/10.1063/1.2195024.
- Wang L.V. Prospects of photoacoustic tomography. Med Phys 2008; 35(12): 5758–5767, http://dx.doi.org/10.1118/1.3013698.
- Li R., Phillips E., Wang P., Goergen C.J., Cheng J.-X. Label-free in vivo imaging of peripheral nerve by multispectral photoacoustic tomography. J Biophoton 2015, http://dx.doi.org/10.1002/jbio.201500004.
- Maslov K., Zhang H.F., Hu S., Wang L.V. Optical-resolution photoacoustic microscopy for in vivo imaging of single capillaries. Opt Lett 2008; 33(9): 929–931, http://dx.doi.org/10.1364/OL.33.000929.
- Wang L., Maslov K., Wang L.V. Single-cell label-free photoacoustic flowoxigraphy in vivo. Proc Natl Acad Sci USA 2013; 110(15): 5759–5764, http://dx.doi.org/10.1073/pnas.1215578110.
- Zhang Y., Cai X., Wang Y., Zhang C., Li L., Choi S.-W., Wang L.V., Xia Y. Noninvasive photoacoustic microscopy of living cells in two and three dimensions through enhancement by a metabolite dye. Angew Chem Int Ed Engl 2011; 50(32): 7359–7363, http://dx.doi.org/10.1002/anie.201101659.
- Sakadžić S., Lee J., Boas D.A., Ayata C. High-resolution in vivo optical imaging of stroke injury andrepair. Brain Res 2015; 1623: 174–172, http://dx.doi.org/10.1016/j.brainres.2015.04.044.
- Wu D., Huang L., Jiang M.S., Jiang H. Contrast agents for photoacoustic and thermoacoustic imaging: a review. Int J Mol Sci 2014; 5(12): 23616–23639, http://dx.doi.org/10.3390/ijms151223616.
- Ricles L.M., Nam S.Y., Treviсo E.A., Emelianov S.Y., Suggs L.J. A dual gold nanoparticle system for mesenchymal stem cell tracking. J Mater Chem B Mater Biol Med 2014; 2(46): 8220–8230, http://dx.doi.org/10.1039/C4TB00975D.
- Nam S.Y., Chung E., Suggs L.J., Emelianov S.Y. Combined ultrasound and photoacoustic imaging to noninvasively assess burn injury and selectively monitor a regenerative tissue-engineered construct. Tissue Eng Part C Methods 2015; 21(6): 557–566, http://dx.doi.org/10.1089/ten.TEC.2014.0306.
- Zharov V.P., Galanzha E.I., Shashkov E.V., Kim J.W., Khlebtsov N.G., Tuchin V.V. Photoacoustic flow cytometry: principle and application for real-time detection of circulating single nanoparticles, pathogens, and contrast dyes in vivo. J Biomed Opt 2007; 12(5): 051503, http://dx.doi.org/10.1117/1.2793746.
- Galanzha E.I., Zharov V.P. Circulating tumor cell detection and capture by photoacoustic flow cytometry in vivo and ex vivo. Cancers (Basel) 2013; 5(4): 1691–1738, http://dx.doi.org/10.3390/cancers5041691.
- Cao F., Drukker M., Lin S., Sheikh A.Y., Xie X., Li Z., Connolly A.J., Weissman I.L., Wu J.C. Molecular imaging of embryonic stem cell misbehavior and suicide gene ablation. Cloning Stem Cells 2007; 9(1): 107–117, http://dx.doi.org/10.1089/clo.2006.0e16.
- Waerzeggers Y., Klein M., Miletic H., Himmelreich U., Li H., Monfared P., Herrlinger U., Hoehn M., Coenen H.H., Weller M., Winkeler A., Jacobs A.H. Multimodal imaging of neural progenitor cell fate inrodents. Mol Imaging Biol 2008; 7(2): 77–91.
- Gaedicke S., Braun F., Prasad S., Machein M., Firat E., Hettich M., Gudihal R., Zhu X., Klingner K., Schüler J., Herold-Mende C.C., Grosu A.L., Behe M., Weber W., Mäcke H., Niedermann G. Noninvasive positron emission tomography and fluorescence imaging of CD133+ tumor stem cells. Proc Natl Acad Sci USA 2014; 111(6): 692–701, http://dx.doi.org/10.1073/pnas.1314189111.
- Hung T.C., Suzuki Y., Urashima T., Caffarelli A., Hoyt G., Sheikh A.Y., Yeung A.C., Weissman I., Robbins R.C., Bulte J.W., Yang P.C. Multimodality evaluation of the viability of stem cells delivered into different zones of myocardial infarction. Circ Cardiovasc Imaging 2008; 1(1): 6–13, http://dx.doi.org/10.1161/CIRCIMAGING.108.767343.
- De Vocht N., Reekmans K., Bergwerf I., Praet J., Hoornaert C., Le Blon D., Daans J., Berneman Z., Van der Linden A., Ponsaerts P. Multimodal imaging of stem cell implantation in the central nervous system of mice. J Vis Exp 2012; (64): e3906, http://dx.doi.org/10.3791/3906.
- Cussó L., Mirones I., Peña-Zalbidea S., García-Vázquez V., García-Castro J., Desco M. Combination of single-photon emission computed tomography and magnetic resonance imaging to track 111in-oxine-labeled human mesenchymal stem cells in neuroblastoma-bearing mice. Mol Imaging 2014; 13: 1–10.
- Jackson J., Chapon C., Jones W., Hirani E., Qassim A., Bhakoo K. In vivo multimodal imaging of stem cell transplantation in a rodent model of Parkinson’s disease. J Neurosci Methods 2009; 183(2): 141–148, http://dx.doi.org/10.1016/j.jneumeth.2009.06.022.
- Wang H., Cao F., De A., Cao Y., Contag C., Gambhir S.S., Wu J.C., Chen X. Trafficking mesenchymal stem cell engraftment and differentiation in tumor-bearing mice by bioluminescence imaging. Stem Cells 2009; 27(7): 1548–1558, http://dx.doi.org/10.1002/stem.81.
- Meleshina A.V., Cherkasova E.I., Shirmanova M.V., Klementieva N.V., Kiseleva E.V., Snopova L.В., Prodanets N.N., Zagaynova E.V. Influence of mesenchymal stem cells on the metastases development in mice in vivo. Stem Cell Res Ther 2015; 6: 15, http://dx.doi.org/10.1186/s13287-015-0003-7.
- Poveshchenko A.F., Poveshchenko O.V., Konenkov V.I. Recent advances in the study of the stem cells migration methods. Vestnik RAMN 2013; 9: 46–51.