Effect of Ozone and Doxorubicin on the Viability and Morphology of Malignant Hepatic Cells
The aim of the investigation was to study the effect of isolated and combined action of ozone-oxygen mixture and doxorubicin on the viability and morphology of normal and malignant human hepatic cells.
Material and Methods. The culture of normal (Chang liver) and malignant (SK-HEP-1) human liver cells were used in the study. Doxorubicin and ozone-oxygen gas mixture were introduced to the culture medium for cell growth. Viability was tested by reduction of MTT tetrazole salts. Morphological investigations were carried out 48 h after the replacement of the cultural medium using DMIL HC inverted microscope (Germany).
Results. Doxorubicin possesses the highest cytotoxicity relative to both normal and malignant human hepatic cells. Ozone alone and in combination with doxorubicin also exerts a marked cytotoxic effect on the viability of the cells and results in irreversible consequences for the structure of the cell elements. The results obtained can be used for selection of ozone doses and ways of ozone introduction in various tumor localizations.
In recent years non-drug methods have been used alongside with surgical and pharmaceutical treatment in medicine. Some of them are applied in oncology for the organism to maintain its own compensatory functions, neuroendocrine and immune systems being in the first place. One of such methods is ozone therapy. Very low doses of ozone are proved to be effective in clinical practice, allowing physicians to correct the organism condition and sustain optimal course of adaptation processes.
Two discoveries of the German scientists may be considered to be preconditions of using ozone in oncology: Warburg (1966) confirmed, that a key premise for tumor development is oxygen deficiency at the cell level, and Varro (1974) revealed peroxide intolerance in tumor cells. In 1980 Sweet et al. presented the evidence of ozone inhibiting effect relative to tumor cells in vitro, having found a weak ability of these cells to compensate the oxygen “burst” caused by ozone in comparison with normal cells, and estimated 90% suppression of malignant cell growth [1].
In 1982 Wolf discovered a dose-dependent antiproliferative effect of ozone [2]. Later Karlic et al. confirmed selective growth inhibition of ovarian and endometrial carcinoma cells by ozone [3]. Zanker and Kroczek found the rise in sensitivity of resistant tumor cells to cytostatic preparations [4].
In the work of Arlan and DeVries [5] increase of survival rate in mice with intertwined carcinoma, injected with ozone-oxygen mixture, was demonstrated. Rodrigues et al. [6] showed antimetastatic effect of ozone. In our experimental studies on laboratory animals with intertwined breast tumor it was shown, that the effect of ozone on malignant cell pathomorphosis is comparable with that of the known doxorubicin preparation [7]. Doxorubicin is an antibiotic of anthracycline series. In recent time doxorubicin is used in the experiments on cell culture in order to develop a method of removing resistance [8, 9]. In our works a marked damaging effect on the tumor was demonstrated by the combination of ozone and doxorubicin. Besides, optimal concentrations of ozone and doxorubicin were determined. To specify the mechanisms of ozone and doxorubicin action the transition from the experiments on the whole animal organism to investigations of the malignant cell culture was necessary.
The aim of the investigation was to study the effect of isolated and combined action of ozone-oxygen mixture and doxorubicin on the viability and morphology of normal and malignant human hepatic cells.
Materials and Methods. Two types of cells were used in the study:
cultured Chang liver cells of normal human liver; cells were cultured in Minimum Essential Medium Eagle (MEM) with Earle’s salts (PanEco, Russia) with addition of 10% fetal bovine serum (PanEco, Russia), optimal density (0.5–1.0)·105 cells/ml;
cells of SK-HEP-1 line (human liver adenocarcinoma, ascitic fluid), with epithelium-like morphology, cultured in MEM Eagle with Earle’s salts (PanEco, Russia) with addition of 10% fetal bovine serum (PanEco, Russia) and 1% of non-essential amino acids (PanEco, Russia), optimal density (2.0–4.0)·106 cells/ml2.
The viability of cultures was maintained in Co2-incubator with 5% CO2 content. The experiment was performed at the passage 3–5. Cells were placed in 48- or 6-well plates. Once 60% growth of the monolayer was reached, the culture medium was replaced by the tested ones.
The tested media were prepared in the following way: a) doxorubicin in the dose of 0.004 mg; b) 150 ml of oxygen; c) 150 ml of ozone-oxygen mixture with 25 mg/L ozone concentration; d) a medium with oxygen + 0.004 mg of doxorubicin; e) a medium with ozone-oxygen mixture + 0.004 mg of doxorubicin. Oxygen was delivered from NewLife Intensity oxygen concentrator (Buffalo, USA). Ozone-oxygen gas mixture was supplied by the ozone generator (Quazar, Russia) with the rate of 1 L/min during 5 min.
48 h later the cultured tested cell medium was removed, the cells were washed with polyphosphate buffer (PBS) (pH=7.4) and 250 g of vercen (0.02%) and tripsin (0.25%) mixture in 3:1 ratio was poured down. After 10 min of incubation in CO2-incubator the cells were pipeted, and 250 ml of 8% formaldehyde were added to each well. Then the number of cells was counted using Septer automatic analyzer (Millipore, Germany).
The viability of cells was tested by the reduction of MTT tetrazolium salts (Sigma, USA) [10]. The method is based on the reaction of reducing yellow tetrazolium salts 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyltetrazolium bromide) by mitochondrial dehydrogenases (oxidoreductase) of the live cells to the blue crystals of formazan [11]. For this purpose the cell medium was removed, the cells were washed in the PBS (pH=7.4), 300 μl of MTT tetrazolium salts (0.25 mg/ml concentration in PBS, pH=7.4) were placed in each well and incubated in CO2-incubator for 30 min. Then the solution was removed and isopropanol was poured on the cells to destroy the cell membranes. In 10 min optical density (A) was measured at 570 and 620 nm wavelengths. Here: 570 nm is a maximum of absorbance of the reduced MTT form; 620 nm is a maximum of tryptophan absorbance proportional to the amount of the protein in the sample, which depends on the number of cells.
The viability (E) was calculated by the following equations:
E=A570–A620;
% of viable cells = (Eexperiment/Econtrol)×100%.
Changes in the optical density of intact cells were considered as a control when studying the effect of oxidizers and doxorubicin on the viability of cells.
Morphological investigations were started 48 h after replacement of the cells in the wells using Leica DMIL HC inverted microscope (Germany). Not less than five non-overlapping fields of view for each culture were examined.
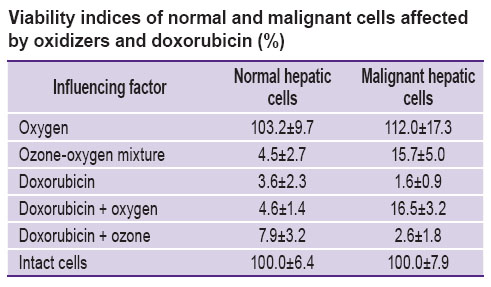
Results. The study of viability indices for normal and malignant hepatic cells (See the Table) showed, that it is directly related to the activity of oxidoreductase enzymes. Decrease in the activity of these enzymes indicates to the marked cytotoxicity of doxorubicin in relation to normal hepatic cells (3.5% viability index) and especially to malignant hepatic cells (1.56% viability index). The analysis of oxidizer action also revealed a directly opposite effect of ozone-oxygen mixture on cell viability. Treatment of the cultural medium by oxygen increased both the activity of normal oxidoreductases (viability index increased to 104%) and malignant cells as well (viability index increased to 115%). It spoke of the fact, that addition of oxygen did not change the composition of the medium for the cell culture and did not interfere with its growth. On the contrary, additional oxygen activated oxidoreductases according to the principle of a feedback. Introduction of ozone-oxygen mixture to the culture medium caused formation of the products of ozone–bioorganic compound interaction (ozonides). These products were likely to damage the cell membranes and to inhibit the activity of oxidoreductases, which manifested itself in the decrease of viability indices (4.54% for normal cells and 15.7% for malignant hepatic cells). The quantitative differences in the viability of normal and malignant cells may be explained by the more powerful antioxidant protection of the malignant cells.
 Viability indices of normal and malignant cells affected by oxidizers and doxorubicin (%) Viability indices of normal and malignant cells affected by oxidizers and doxorubicin (%)
|
Combined usage of doxorubicin and oxygen showed, that application of two agents exerted a greater effect on the normal cells, rather than on the malignant ones, having more intensive antioxidant protective system. The combination of doxorubicin and ozone was accompanied by the decrease of viability of normal (up to 7.9%) and malignant cells (up to 2.6%). The difference of these values from those obtained in case of isolated introduction of doxorubicin and ozone seems to be connected with the interaction of these factors and components of the cells, including antioxidants.
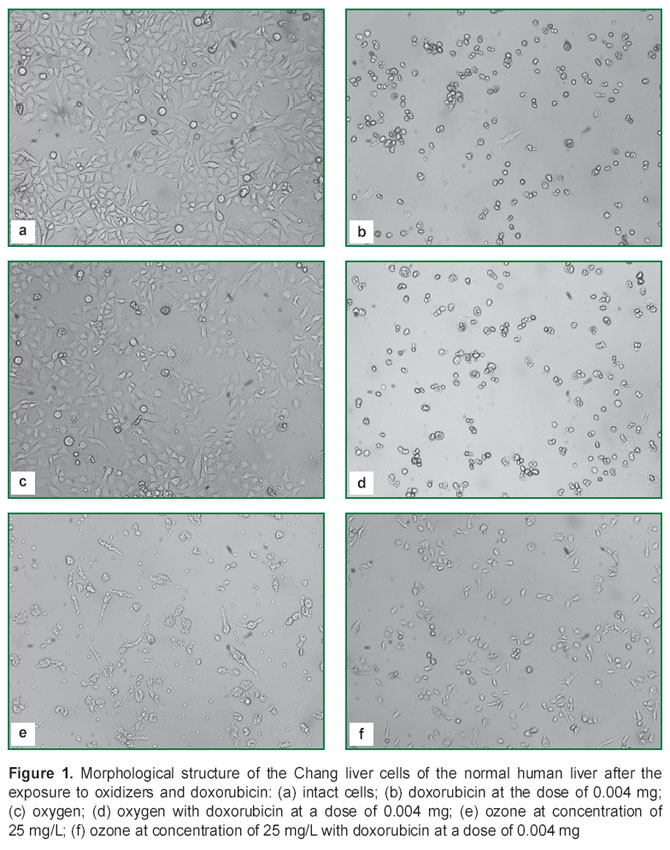
Investigation of the morphological structure of the cells of both selected lines (Figures 1, 2) showed, that oxygen did not exert toxic effect on the cell lines (Figures 1 (c) and 2 (c)) 48 h after the replacement of the culture medium. The cell structure remained unchanged, condensation of chromatin typical for apoptosis was not observed. Actively divided cells were well seen in the culture, as well as separate cellular elements, detached from the substrate and freely floating in the cell medium (one of the stages of monolayer development). In the Chang liver culture a small amount of cells with a changed morphology was found, the cell contours were irregular, chromatin was changed, but the number of morphological structure changes was not significant. In spite of the fact, that the increase of oxygen concentration for the cell lines is not physiologically justified and is considered as an unfavorable factor, no changes of the morphological structure and cell viability were revealed even at a maximal saturation of the culture medium.
Application of doxorubicin in the culture of Chang liver cell line (Figure 1 (b)) in the selected concentration resulted in significant alterations of the cell morphology. Loss of the characteristic cell structure was observed, the cells lost their form, detached from the substrate, diminished in size, evident alterations of the nuclear apparatus and all organelles were determined. These changes were characteristic of the dead cells. Addition of oxygen did not influence the effects caused by doxorubicin in the concentration of 0.004 mg (Figure 1 (d)).
When studying morphology after 48-hour of culturing in the ozonized medium, cellular elements, indicating to the terminal stage of apoptosis, and the elements with the destroyed membranes, were found (Figure 1 (e)).
When ozone was used together with doxorubicin, a cytostatic effect was revealed (Figure 1 (f)). Separate cellular elements with the morphology typical for the given line were found, cells with the damaged membranes were present, dividing cells were not detected. A monolayer was not formed during 48 h after the exposure (as in the control series). However, the number of dead cells and those detached from the substrate was relatively small.
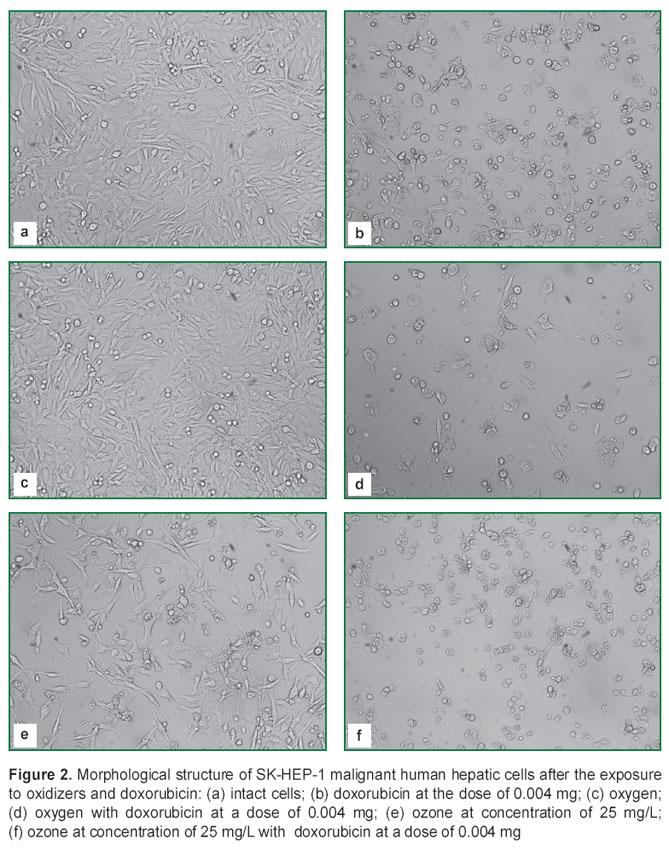
In the cultures of SK-HEP-1 cell line irreversible changes in the majority of the cells were caused by the addition of the cytostatic (Figure 2 (b)): they lost their form, chromatin condensed, mass mortality of the cells was observed. But the form of the small part of the non-dividing cells remained spindle-shaped, typical for the given line. Dividing cells were not detected.
Combined application of doxorubicin and oxygen did not influence the effects caused by cytostatics (Figure 2 (d)). Usage of the ozonized medium caused a marked cytostatic effect (Figure 2 (e)), as the rate of obtaining the monolayer is lower. However, dividing cells were detected along with a great number of spindle-shaped cells 48 h after the addition of the ozonized medium to the culture.
Combined application of doxorubicin and ozone (Figure 2 (f)) led to irreversible consequences for the structure of the cellular elements. Cells with a normal form were not found: cellular bodies were rounded, chromatin condensed, membranes were damaged. Cellular “shadows” were encountered — as a terminal stage of necrotic death.
The morphological analysis conducted demonstrated, that combined application of doxorubicin and ozone intensified the cytostatic effect of each of the given substances. The cells of liver adenocarcinoma were more resistant to both ozone and doxorubicin than the cells of normal liver. Replacement of the culture medium by the medium saturated with oxygen did not influence the morphology of either liver adenocarcinoma cells or normal liver lines.
Thus, the investigation of viability of liver cells of the selected lines and the data of their morphological structure changes convincingly testify, that doxorubicin possesses marked cytotoxicity relative to the normal hepatic cells and especially to the malignant cells of the liver. Application of oxygen alone does not alter the composition of the medium for the cell culture and does not interfere with cell proliferation. Combined application of doxorubicin and oxygen causes more prominent effect on the normal cells rather than malignant ones, which have a more pronounced antioxidant protection system. Introduction of ozone-oxygen mixture to the culture medium leads to the decrease of viability indices for malignant hepatic cells. Combination of doxorubicin and ozone is accompanied by the reduction of viability of normal and malignant cells.
Conclusion. Combined application of ozone and doxorubicin causes cytotoxic effect on the viability of normal and malignant human hepatic cells, results in irreversible consequences for the structure of the cellular elements. Effect of ozone is comparable with the action of doxorubicin, enabling oncologists to choose the ways of ozone introduction — separately or in combination with doxorubicin.
Study Funding and Conflicts of Interest. The work was not supported by any financial sources. The authors declare no conflicts of interest related to the study.
References
- Sweet F., Kao M.S., Lee S.C., Hagar W.L., Sweet W.E. Ozone selectively inhibits growth of human cancer cells. Science 1980; 209(4459): 931–933, http://dx.doi.org/10.1126/science.7403859.
- Wolf H.H. Das medizinische Ozon [Medical ozone]. Heidelberg; 1982; 250 p.
- Karlic H., Kucera H., Metka M., Schönbauer M., Söregi G. Effect of ozone and ionizing radiation on an in vitro model — a pilot study of 4 gynecologic tumors. Strahlenther Onkol 1987; 163(1): 37–42.
- Zanker K.S., Kroczek R. The mystery of molecule-ozone: antiproliferative, immunmodulative, synergistic to chemotherapy and carcinogenetic carcinogenic. In: IX Congress of Ozone. New York; 1989; p. 55–68.
- Arnan M., DeVries L.E. Effect of ozone/oхygen gas miхture directly injected into the mammary carcinoma of the female C3H/HEJ mice. In: Medical applications of ozone 1983; p. 101–107.
- Rodriguez Y., Bello J.L., Menendez S., et al. Antitumor activity of ozone. Experimental research. Ozone News 1997; 25(3): 50.
- Alyasova A.V., Kontorshickova K.N., Terentiev I.G., Ivanova I.P., Kuznetsov S.S., Sazanov A.I. Influence of the ozonized physiologic salt solution low therapeutic concentrations on a tumor therapeutic pathomorphosis in experiment. Sovremennye tehnologii v medicine 2010; 4: 27–32.
- Boyko N.N., Sen’kiv Yu.V., Shlyakhtina E.A., Klyuchivskaya O.Yu., Skorokhid N.R., Mitina N.E., Skorokhoda T.V., Moskvin M.M., Zaichenko A.S., Stoyka R.S. Action of doxorubicin delivered to tumor cells in vitro and in vivo by novel nanoscale oligoelectrolytic carrier. Biotechnologia Acta 2013; 6(3): 53–62.
- Zamulaeva I.A., Pronyushkina K.A., Matchuk O.N., Yabbarov N.G., Nikol’skaya E.D., Kondrasheva I.G. Combined effect of ionizing radiation and dendritic polymers, loaded by doxorubicin on the breast cancer cells of MCF-7 line. Radiatsionnaya biologiya. Radioekologiya 2015; 6: 591–597.
- Anikina L.V., Pukhov S.A., Dubrovskaya E.S., Afanas’eva S.V., Klochkov S.G. Comparative definition of cell viability by MTT and Resazurin. Fundamentalnie issledovania 2014; 12(part 7): 1423–1427.
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 1983; 65(1–2): 55–63, http://dx.doi.org/10.1016/0022-1759(83)90303-4.