Pathomorphological Assessment Method of Myocardial Infarction Age
The aim of the investigation was to develop a simple and available for practical application method of pathomorphological assessment of myocardial infarction age with a wide range of registered terms.
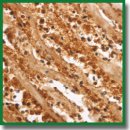
Materials and Methods. Specimens of the experimental myocardial infarction zone (a model of diathermocoagulation of the rat periconical interventricular artery; n=50) in the period from 2 h to 30 days, and post-mortem specimens of 30 men died of transmural myocardial infarction and post-infarction cardiosclerosis in the period from 6 h to 30 days were immunohistochemically investigated using antibodies to the matrix metalloproteinase 9 (MMP9).
Results. It has been established that localization of MMP9 in the infarction zone enables determining with a sufficiently high precision the time that passed from infarction onset. In fatal outcomes developed during several hours after infarction, the neutrophil cytoplasm in the infarction zone had intensive color. On day 1–2 a bright color of the extracellular matrix was noted. In later terms (days 3–21) staining of the fibroblastic cell line in the border zone (with maximum on days 7–14) was observed. The dynamics of MMP9 content and its localization in the myocardium of the dead patients corresponds to the data obtained in the experimental investigations.
Conclusion. The proposed method allows pathologists to clearly differentiate myocardial infarction age using pathomorphological investigations.
- Zech W.D., Schwendener N., Persson A., Warntjes M.J., Jackowski C. Postmortem MR quantification of the heart for characterization and differentiation of ischaemic myocardial lesions. Eur Radiol 2015; 25(7): 2067–2073, https://doi.org/10.1007/s00330-014-3582-2.
- Kostin S., Hein S., Arnon E., Scholz D., Schaper J. The cytoskeleton and related proteins in the human failing heart. Heart Fail Rev 2000; 5(3): 271–280, https://doi.org/10.1023/A:1009813621103.
- Kakturskiy L.V., Rybakova M.G., Kuznetsova I.A. Sudden cardiac death (morphological diagnosis). Biblioteka patologoanatoma 2008; 100: 45–61.
- Thomsen H., Held H. Immunohistochemical detection of C5b-9(m) in myocardium: an aid in distinguishing infarction-induced ischemic heart muscle necrosis from other forms of lethal myocardial injury. Forensic Sci Int 1995; 71(2): 87–95, https://doi.org/10.1016/0379-0738(94)01640-q.
- Campobasso C.P., Dell’Erba A.S., Addante A., Zotti F., Marzullo A., Colonna M.F. Sudden cardiac death and myocardial ischemia indicators: a comparative study of four immunohistochemical markers. Am J Forensic Med Pathol 2008; 29(2): 154–161, https://doi.org/10.1097/PAF.0b013e318177eab7.
- Hu B.J., Chen Y.C., Zhu J.Z. Study on the specificity of fibronectin for post-mortem diagnosis of early myocardial infarction. Med Sci Law 2002; 42(3): 195–199, https://doi.org/10.1177/002580240204200303.
- Sapouna R., Gourgiotis D., Athanaselis S., Papadodima S., Spiliopoulou C. Diagnostic value of cardiac troponin I in postmortem diagnosis of myocardial infarction. Am J Forensic Med Pathol 2013; 34(2): 139–141, https://doi.org/10.1097/PAF.0b013e3182880aa1.
- Meng X., Ming M., Wang E. Heart fatty acid binding protein as a marker for postmortem detection of early myocardial damage. Forensic Sci Int 2006; 160(1): 11–16, https://doi.org/10.1016/j.forsciint.2005.08.008.
- Hashmi S., Al-Salam S. Loss of dystrophin staining in cardiomyocytes: a novel method for detection early myocardial infarction. Int J Clin Exp Pathol 2013; 6(2): 249–257.
- Ouyang J., Guzman M., Desoto-Lapaix F., Pincus M.R., Wieczorek R. Utility of desmin and a Masson’s trichrome method to detect early acute myocardial infarction in autopsy tissues. Int J Clin Exp Pathol 2009; 3(1): 98–105.
- Ortmann C., Pfeiffer H., Brinkmann B. A comparative study on the immunohistochemical detection of early myocardial damage. Int J Legal Med 2000; 113(4): 215–220, https://doi.org/10.1007/s004149900094.
- Shurygin M.G., Shurygina I.A., Dremina N.N. Dynamics of vasoendothelial growth factor and fibroblast growth factor in experimental cardiac infarction. Byulleten’ Vostochno-Sibirskogo nauchnogo tsentra Sibirskogo otdeleniya Rossiyskoy akademii meditsinskikh nauk 2007; 6: 169–174.
- Shurygina I.A., Shurygin M.G., Ayushinova N.I. Expression of apoptosis markers in adhesions in the abdominal cavity under the experimental conditions. Vestnik Rossiyskoy akademii meditsinskikh nauk 2014; 69(5–6): 29–33.
- Ruiz-Villalba A., Simón A.M., Pogontke C., Castillo M.I., Abizanda G., Pelacho B., Sánchez-Domínguez R., Segovia J.C., Prósper F., Pérez-Pomares J.M. Interacting resident epicardium-derived fibroblasts and recruited bone marrow cells form myocardial infarction scar. J Am Coll Cardiol 2015; 65(19): 2057–2066, https://doi.org/10.1016/j.jacc.2015.03.520.
- Shurygina I.A., Shurygin M.G., Dremina N.N., Kanya O.V. Sposob patomorfologicheskogo opredeleniya davnosti nastupleniya infarkta miokarda [Method for pathomorphological determination of prescription of myocardial infarction]. Patent RU 2518333 С1. 2012.










