Replacement of Osteochondral Defects of Major Joints in Experiment
The aim of the study was to develop a novel treatment method of knee osteochondral defects consisting in injecting platelet-rich plasma and crushed hyaline cartilage under a collagen membrane, and assess the technique in experiment.
Materials and Methods. A prospective study was carried out on small cattle animals, 30 in number, aged 1.5–3 years weighing 20–30 kg. All subjects got a full-thickness defect to the subchondral bone, 4.5 mm in diameter. As a control, one of the joint defects was not replaced. Due to a replacement method, all animals were divided into three groups. One group animals underwent the replacement according to the developed technique: there were used an extracellular collagen matrix and the body resources (platelet-rich plasma and crushed autologous cartilage).
Results. The results were assessed 1 month and 3 months after surgery analyzing the type and degree of defect filling. Best results were found in the group, where a defect was covered by an extracellular collagen matrix with platelet-rich plasma and crushed autologous cartilage. The results of the no replacement group were comparable with the findings of other researchers, according to which osteochondral defects almost have no self-regeneration.
Conclusion. The suggested replacement technique for osteochondral defect using extracellular collagen matrix, autologous cartilage, and platelet-rich plasma is less aggressive compared to autochondroplasty, and the obtained results are more stable compared to microfracture or tunneling.
Introduction
One of most common complaints of patients seeing an orthopedist is joint pain [1, 2]. According to some reports, 60% of such patients are found to have chondromalacia of hyaline cartilage of varying severity [3]. A range of conservative therapy is wide; however, its efficiency is still insufficient [4–6]. Against this background, surgical techniques are being rapidly elaborated [7–10].
One of the easiest methods suggested as early as in 1960s is tunneling. Despite the fact that this method is used now, long-term results are estimated as poor [11, 12]. Currently, the most popular surgical technique to treat chondromalacia is microfracture. However, it should be noted that the technique has some drawbacks, such as instability of newly formed tissue to loads, and progression of degenerative and dystrophic processes [12, 13].
The aim of the study was to develop a novel treatment method of knee osteochondral defects consisting in introducing platelet-rich plasma and crushed hyaline cartilage under collagen membrane, and assess the technique in experiment.
Materials and Methods
A prospective study was performed on small cattle animals — sheep (n=30) aged 1.5–3 years, weighing 20–30 kg. The choice of experimental animals was due to the similarity of human and sheep knee joints, and therefore, the feasibility of creating an osteochondral defect model identical to that of human. The animals respond well to anesthesia and can be used as experimental animals in Russia. The research was performed in compliance with “Animal Cruelty Protection Act” dated 01.12.1999.
Sheep were divided into three groups, 10 animals in each group (20 knee joints) depending on the osteochondral defect replacement method. In each group each animal underwent the joint defect replacement technique and had one unreplaced defect (control). A defect, 4.5 mm in diameter, was formed up to the subchondral bone, on the load-bearing surface of femoral medial condyle. Group 1 involved the animals with a knee defect replaced by a collagen membrane; group 2 animals were injected platelet-rich plasma (PRP) under a collagen membrane; and in group 3 animals, additionally, crushed hyaline cartilage from a non-load-bearing joint surface was used.
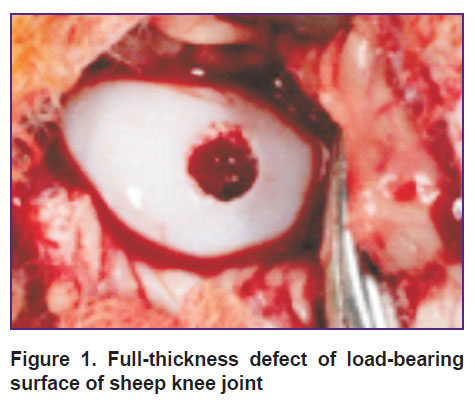
Rometar 2% solution, 0.5 ml, was used as anesthetic. The procedures were performed on animals in the lateral position. After trimming wool, the intervention area was treated with antiseptic solution thrice. The bent limb was immobilized. The surgical approach was 4 cm lateral to the patellar ligament. Then subcutaneous fat was cut providing an access to the knee joint. An osteochondral defect, 4.5 mm in diameter, was formed by a drill for mosaic autochondroplasty (Figure 1). After that the defect was replaced using one of the above mentioned techniques. A wound was sutured layer-by-layer leaving no drainage in the postoperative wound.
|
Figure 1. Full-thickness defect of load-bearing surface of sheep knee joint |
All sheep were kept in the vivarium suitable for animal management, in accordance with RF legislation. The load on a limb was allowed immediately after surgery.
The obtained material was visually and morphologically studied by light microscopy.
For morphological study the material was fixed in 10% formaldehyde and dried out.
The paper presents the results obtained in the animals with no defect replacement (control) and group 3 animals 3 months after the surgery.
Results
It should be noted that none group animals had any inflammatory changes in the joint. The wounds healed by primary intention.
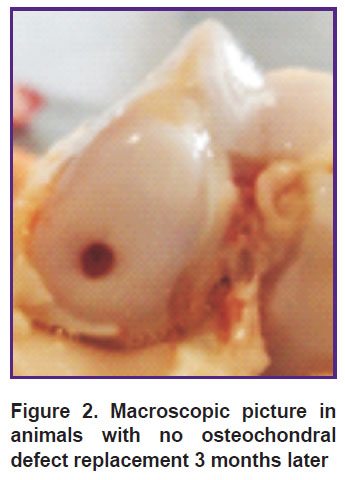
Three months after the operation the animals with no defect replacement had a “minus tissue” effect, the osteochondral defect base being erosive, almost not filled; the border between the defect area and the proper hyaline cartilage was clearly observed (Figure 2).
|
Figure 2. Macroscopic picture in animals with no osteochondral defect replacement 3 months later |
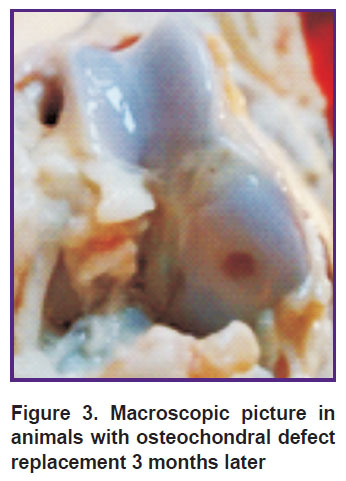
The animals in the group with a defect replaced by a collagen membrane, PRP and crushed auto-cartilage also had “minus tissue” composing 2/3 of defect thickness. Osteochondral defect margins were smooth, no erosive surface being determined; while the boundary between the newly formed tissue and the hyaline joint cartilage was less clear (Figure 3).
|
Figure 3. Macroscopic picture in animals with osteochondral defect replacement 3 months later |
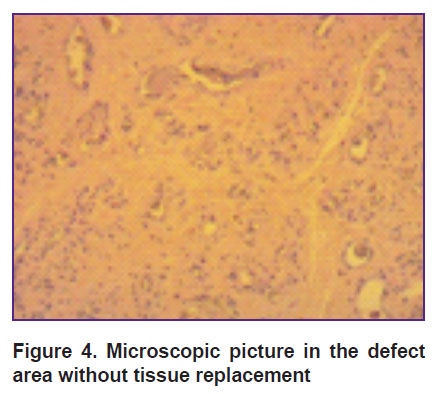
Morphologically, 3 months later, the animals with no defect replacement had a thin discontinuous layer of a newly formed tissue. Along the defect periphery, cartilaginous tissue was significantly thicker than in the base, where the thickness was 1/3 of that of the newly formed defect (Figure 4).
|
Figure 4. Microscopic picture in the defect area without tissue replacement |
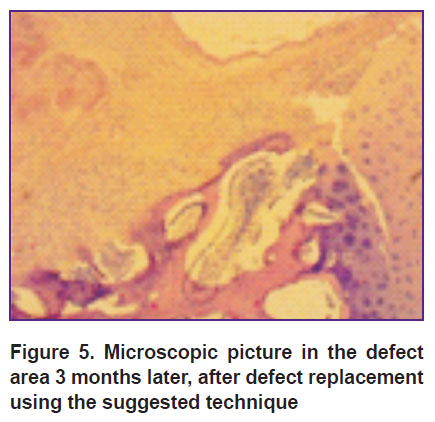
Group 3 animals 3 months after the surgery had a more stable layer. Hyaline-like tissue along the defect periphery was significantly thicker than that one deep in the tissue, where its thickness was 2/3 of the defect thickness. Trabeculas of the bone and the subchondral bone plate adjoining the defect were formed not along the entire base. The plates were thickened, the intercellular substance was found in large quantities, trabeculas of the bone being located chaotically (Figure 5).
|
Figure 5. Microscopic picture in the defect area 3 months later, after defect replacement using the suggested technique |
Discussion
There is growing recognition of the fact that osteochondral defects of major joints require surgery [14]. In most cases such defects are detected by MRI that enables to choose an adequate replacement technique. Currently, arthroscopic debridement of the damaged hyaline cartilage area works well, with defect microfracture being used additionally [15, 16]. However, according to some authors, the method will fail to replace the defect completely, and the resulting restoration rapidly lyses [17, 18]. We believe that one of the most optimal replacement techniques for such defects is mosaic autochondroplasty. It enables to entirely replace a defect in the load-bearing joint part, although a donor’s area can cause pain syndrome and induce rapid progression of degenerative and dystrophic joint diseases [19].
Currently, there are rapidly advancing methods of cultured chondrocytes and their implantation in a defect area on the matrix or under the material restraining the defect area from the joint cavity. The methods are autologous chondrocyte implantation (ACI) and autologous matrix-induced chondrogenesis (AMIC). Both techniques demonstrate good results, however, ACI involves 2 operations to be performed, and AMIC is costly [19, 20].
The methods using mesenchymal stem cells are promising, however, they are not impeccable either
[21, 22].
The technique we suggest uses a collagen membrane and body resources, namely: platelet-rich plasma and crushed auto-cartilage. It is a one-step procedure in contrast to ACI, and it is less aggressive compared to mosaic autochondroplasty. The findings we got on experimental animals with no defect replacement are comparable with those of other researchers. The animals with an osteochondral defect replaced according to the suggested technique had the most optimistic results: the defect regenerated well and closed by 2/3, and morphologically, we could trace the architectonics of hyaline-like cartilaginous tissue that can suggest good results.
Conclusion
Three months after using the method of replacing an osteochondral defect by a collagen membrane with adding platelet-rich plasma and crushed auto-cartilage we can suggest good intermediate results. The technique enabled to achieve nearly complete replacement of the defect and recover a regenerate with the architectonics typical for healthy hyaline cartilage.
Study funding. The study was supported by Stavropol State Medical University.
Conflicts of interest. The authors have no conflicts of interest related to the present study.
References
- Bozhokin M.S., Bozhkova S.A., Netylko G.I. Possibilities of current cellular technologies for articular cartilage repair (analytical review). Travmatologiya i ortopediya Rossii 2016; 22(3): 122–134.
- Belousova T.E., Karpova Zh.Y., Kovalyova M.V. The influence of low-frequency magnetophototherapy on the dynamics of electromyographic indexes in the rehabilitation of patients with combined pathology of the spine and major joints. Sovremennye tehnologii v medicine 2011; 3(2): 77–80.
- Yezhov M.Y., Yezhov I.Y., Kashko A.K., Kayumov A.Y., Zykin A.A., Gerasimov S.A. Unresolved issues of the cartilage and the bone regeneration (review). Uspekhi sovremennogo estestvoznaniya 2015; 5: 126–131.
- Chichasova N.V. Clinical rationale for the use of various teraflex formulations in osteoarthrosis. Sovremennaya revmatologiya 2010; 4(4): 59–64.
- Andia I., Abate M. Knee osteoarthritis: hyaluronic acid, platelet-rich plasma or both in association? Expert Opin Biol Ther 2014; 14(5): 635–649, https://doi.org/10.1517/14712598.2014.889677.
- Chang K.-V., Hung C.-Y., Aliwarga F., Wang T.-G., Han D.-S., Chen W.-S. Comparative effectiveness of platelet-rich plasma injections for treating knee joint cartilage degenerative pathology: a systematic review and meta-analysis. Arch Phys Med Rehabil 2014; 95(3): 562–575, https://doi.org/10.1016/j.apmr.2013.11.006.
- Dhollander A., Moens K., Van der Maas J., Verdonk P., Almqvist K.F., Victor J. Treatment of patellofemoral cartilage defects in the knee by autologous matrix-induced chondrogenesis (AMIC). Acta Orthop Belg 2014; 80(2): 251–259.
- Teplyashin A.S., Sharifullina S.Z., Chupikova N.I., Sepiashvili R.I. The perspective of use of multipotent mesenchymal stromal cells isolated from bone marrow and adipose tissue in regulation of regeneration of bone and cartilage tissues. Allergologiya i immunologiya 2015; 16(1): 138–148.
- Kozadaev M.N. Use of polycaprolactone-based matrices to stimulate the articular cartilage regeneration in experimental conditions. Teoreticheskie i prikladnye aspekty sovremennoy nauki 2014; 3–2: 128–130.
- Ulstein S., Årøen A., Røtterud J.H., Løken S., Engebretsen L., Heir S. Microfracture technique versus osteochondral autologous transplantation mosaicplasty in patients with articular chondral lesions of the knee: a prospective randomized trial with long-term follow-up. Knee Surg Sports Traumatol Arthrosc 2014; 22(6): 1207–1215, https://doi.org/10.1007/s00167-014-2843-6.
- Pridie K.H., Gordon G. A method of resurfacing osteoarthritic knee joints. J Bone Joint Surg 1959; 41(3): 618–619.
- Ewers B.J., Dvoracek-Driksna D., Orth M.W., Haut R.C. The extent of matrix damage and chondrocyte death in mechanically traumatized articular cartilage explants depends on rate of loading. J Orthop Res 2001; 19(5): 779–784, https://doi.org/10.1016/s0736-0266(01)00006-7.
- Shevtsov V.I., Makushin V.D., Stupina T.A., Stepanov M.A. The experimental aspects of studying articular cartilage reparative regeneration under subchondral zone tunneling with autologous bone marrow infusion. Geniy ortopedii 2010; 2: 5–10.
- Sovetnikov N.N., Kalsin V.A., Konoplyannikov M.A., Mukhanov V.V. Cell technologies and tissue engineering in the treatment of articular chondral defects. Klinicheskaya praktika 2013; 1: 52–66.
- Steadman J.R., Briggs K.K., Rodrigo J.J., Kocher M.S., Gill T.J., Rodkey W.G. Outcomes of microfracture for traumatic chondral defects of the knee: average 11-year follow-up. Arthroscopy 2003; 19(5): 477–484, https://doi.org/10.1053/jars.2003.50112.
- Kreuz P.C., Erggelet C., Steinwachs M.R., Krause S.J., Lahm A., Niemeyer P., Ghanem N., Uhl M., Südkamp N. Is microfracture of chondral defects in the knee associated with different results in patients aged 40 years or younger? Arthroscopy 2006; 22(11): 1180–1186, https://doi.org/10.1016/j.arthro.2006.06.020.
- Ulstein S., Årøen A., Røtterud J.H., Løken S., Engebretsen L., Heir S. Microfracture technique versus osteochondral autologous transplantation mosaicplasty in patients with articular chondral lesions of the knee: a prospective randomized trial with long-term follow-up. Knee Surg Sports Traumatol Arthrosc 2014; 22(6): 1207–1215, https://doi.org/10.1007/s00167-014-2843-6.
- Knutsen G., Engebretsen L., Ludvigsen T.C., Drogset J.O., Grøntvedt T., Solheim E., Strand T., Roberts S., Isaksen V., Johansen O. Autologous chondrocyte implantation compared with microfracture in the knee. A randomized trial. J Bone Joint Surg Am 2004; 86(3): 455–464.
- Jacobi M., Villa V., Magnussen R.A., Neyret P. MACI — a new era? Sports Med Arthrosc Rehabil Ther Technol 2011; 3(1), https://doi.org/10.1186/1758-2555-3-10.
- Khan W.S., Johnson D.S., Hardingham T.E. The potential of stem cells in the treatment of knee cartilage defects. Knee 2010; 17(6): 369–374, https://doi.org/10.1016/j.knee.2009.12.003.
- Mafi R. Sources of adult mesenchymal stem cells applicable for musculoskeletal applications — a systematic review of the literature. Open Orthop J 2011; 5(1): 242–248, https://doi.org/10.2174/1874325001105010242.
- Zhai L.J., Zhao K.Q., Wang Z.Q., Feng Y., Xing S.C. Mesenchymal stem cells display different gene expression profiles compared to hyaline and elastic chondrocytes. Int J Clin Exp Med 2011; 4(1): 81–90.










