Cell Model for Testing Pharmaceuticals Targeting Human PD-L1
The aim of this study was to create and evaluate a cell model designed for in vitro and in vivo testing of anti-human PD-L1 therapeutic and diagnostic agents’ specificity.
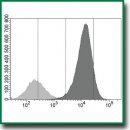
Materials and Methods. Genetically modified cells expressing human PD-L1 (strain CT26-PD-L1) were obtained by retroviral transduction of murine CT26 carcinoma cells. PD-L1 gene activity was assessed by real-time PCR, and PD-L1 expression on cells was identified by flow cytometry. Cells were tested using recombinant single-domain human anti-PD-L1 antibodies (nanoantibodies) conjugated with radioisotopes 68Ga or 177Lu. Immunoreactive fraction and cell internalization of the radioconjugates were evaluated in vitro. For in vivo experiments CT26-PD-L1 cells were transplanted into mice, radioimmunoconjugates were injected 9–14 days later, in 1–48 h the tumors were retrieved and subjected to direct radiometry. Intact CT26 cells not expressing the antigen served as a control.
Results. CT26-PD-L1 strain of murine tumor cells expressing human membrane PD-L1 was created. When transplanted into intact BALB/c mice or sublethally irradiated F1(DBA×BALB/c) mice, these cells formed tumors. Thus, a significant advantage of the model was the possibility of in vivo testing of human PD-L1-affinity agents using animals under conventional vivarium conditions. When radioimmunoconjugates were administered to tumor bearing mice, radionuclides accumulated in tumors generated from the transplanted CT26-PD-L1 cells, but not CT26 cells. CT26-PD-L1 cells internalized anti-PD-L1 nanobodies in vitro. Due to a high density of target molecules, CT26-PD-L1 cells allowed both to confirm pharmaceuticals’ specificity and to quantify the target-binding fraction of conjugates in a single test.
Conclusion. The created cells are the first genetically engineered cells designed to evaluate affinity of anti-human PD-L1 therapeutic and diagnostic agents in Russia. Test results confirmed the model suitability for in vitro and in vivo testing of the specificity of pharmaceuticals targeting human PD-L1.
- Freeman G.J., Long A.J., Iwai Y., Bourque K., Chernova T., Nishimura H., Fitz L.J., Malenkovich N., Okazaki T., Byrne M.C., Horton H.F., Fouser L., Carter L., Ling V., Bowman M.R., Carreno B.M., Collins M., Wood C.R., Honjo T. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med 2000; 192(7): 1027–1034, https://doi.org/10.1084/jem.192.7.1027.
- Carter L., Fouser L.A., Jussif J., Fitz L., Deng B., Wood C.R., Collins M., Honjo T., Freeman G.J., Carreno B.M. PD-1:PD-L inhibitory pathway affects both CD4(+) and CD8(+) T cells and is overcome by IL-2. Eur J Immunol 2002; 32(3): 634–643, https://doi.org/10.1002/1521-4141 (200203)32:3634::AID-IMMU6343.0.CO;2-9.
- Guleria I., Khosroshahi A., Ansari M.J., Habicht A., Azuma M., Yagita H., Noelle R.J., Coyle A., Mellor A.L., Khoury S.J., Sayegh M.H. A critical role for the programmed death ligand 1 in fetomaternal tolerance. J Exp Med 2005; 202(2): 231–237, https://doi.org/10.1084/jem.20050019.
- Brown J.A., Dorfman D.M., Ma F.R., Sullivan E.L., Munoz O., Wood C.R., Greenfield E.A., Freeman G.J. Blockade of programmed death-1 ligands on dendritic cells enhances T cell activation and cytokine production. J Immunol 2003; 170(3): 1257–1266, https://doi.org/10.4049/jimmunol.170.3.1257.
- Iwai Y., Ishida M., Tanaka Y., Okazaki T., Honjo T., Minato N. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc Natl Acad Sci U S A 2002; 99(19): 12293–12297, https://doi.org/10.1073/pnas.192461099.
- Dong H., Strome S.E., Salomao D.R., Tamura H., Hirano F., Flies D.B., Roche P.C., Lu J., Zhu G., Tamada K., Lennon V.A., Celis E., Chen L. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med 2002; 8(8): 793–800, https://doi.org/10.1038/nm730.
- Wang X., Teng F., Kong L., Yu J. PD-L1 expression in human cancers and its association with clinical outcomes. Onco Targets Ther 2016; 9: 5023–5039, https://doi.org/10.2147/OTT.S105862.
- Karpenko T.D., Kozlov N.A., Lubchenko L.N., Rotobelskaya L.E., Bagrova S.G., Safronova V.M., Laktionov K.K., Bychkov M.B. Analysis of protein expression of PD-L1 in malignant pleural mesothelioma. Russian Journal of Oncology 2018; 23(1): 4–9, https://doi.org/10.18821/1028-9984-2018-23-1-4-9.
- Pinevich A.A., Vartanyan N.L., Kiseleva L.N., Bode I.I., Krutetskaya I.Yu., Kartashev A.V., Makarov V.E., Poneza T.E., Smirnov I.V., Samoilovich M.P. PD-L1 and PD-L2 gene expression in human glioblastoma cells resistant to chemo- and radiotherapy. Meditsinskaya immunologiya 2023; 25(3): 605–610, https://doi.org/10.15789/1563-0625-PLA-2693.
- Wang J., Xu Y., Rao X., Zhang R., Tang J., Zhang D., Jie X., Zhu K., Wang X., Xu Y., Zhang S., Dong X., Zhang T., Yang K., Xu S., Meng R., Wu G. BRD4-IRF1 axis regulates chemoradiotherapy-induced PD-L1 expression and immune evasion in non-small cell lung cancer. Clin Transl Med 2022; 12(1): e718, https://doi.org/10.1002/ctm2.718.
- Peng J., Hamanishi J., Matsumura N., Abiko K., Murat K., Baba T., Yamaguchi K., Horikawa N., Hosoe Y., Murphy S.K., Konishi I., Mandai M. Chemotherapy induces programmed cell death-ligand 1 overexpression via the nuclear factor-κB to foster an immunosuppressive tumor microenvironment in ovarian cancer. Cancer Res 2015; 75(23): 5034–5045, https://doi.org/10.1158/0008-5472.CAN-14-3098.
- Zanello A., Bortolotti M., Maiello S., Bolognesi A., Polito L. Anti-PD-L1 immunoconjugates for cancer therapy: are available antibodies good carriers for toxic payload delivering? Front Pharmacol 2022; 13: 972046, https://doi.org/10.3389/fphar.2022.972046.
- Pisaneschi F., Viola N.T. Development and validation of a PET/SPECT radiopharmaceutical in oncology. Mol Imaging Biol 2022; 24(1): 1–7, https://doi.org/10.1007/s11307-021-01645-6.
- Sharma S.K., Lyashchenko S.K., Park H.A., Pillarsetty N., Roux Y., Wu J., Poty S., Tully K.M., Poirier J.T., Lewis J.S. A rapid bead-based radioligand binding assay for the determination of target-binding fraction and quality control of radiopharmaceuticals. Nucl Med Biol 2019; 71: 32–38, https://doi.org/10.1016/j.nucmedbio.2019.04.005.
- Avrov K.O., Shatik S.V., Zaitsev V.V., Al-Shehadat R.I., Shashkova O.A., Terekhina L.A., Malakhov I.S., Samoylovich M.P. Application of magnetic particles for fast determination of immunoreactive fraction of 68Ga-labelled VHH antibodies to PD-L1. Sovremennye tehnologii v medicine 2023; 15(3): 26–33, https://doi.org/10.17691/stm2023.15.3.03.
- Stribbling S.M., Ryan A.J. The cell-line-derived subcutaneous tumor model in preclinical cancer research. Nat Protoc 2022; 17(9): 2108–2128, https://doi.org/10.1038/s41596-022-00709-3.
- Huang A., Peng D., Guo H., Ben Y., Zuo X., Wu F., Yang X., Teng F., Li Z., Qian X., Qin F.X. A human programmed death-ligand 1-expressing mouse tumor model for evaluating the therapeutic efficacy of anti-human PD-L1 antibodies. Sci Rep 2017; 7: 42687, https://doi.org/10.1038/srep42687.
- Yang Y., Wang C., Wang Y., Sun Y., Huang X., Huang M., Xu H., Fan H., Chen D., Zhao F. Dose escalation biodistribution, positron emission tomography/computed tomography imaging and dosimetry of a highly specific radionuclide-labeled non-blocking nanobody. EJNMMI Res 2021; 11(1): 113, https://doi.org/10.1186/s13550-021-00854-y.
- Qin S., Yu Y., Guan H., Yang Y., Sun F., Sun Y., Zhu J., Xing L., Yu J., Sun X. A preclinical study: correlation between PD-L1 PET imaging and the prediction of therapy efficacy of MC38 tumor with 68Ga-labeled PD-L1 targeted nanobody. Aging (Albany NY) 2021; 13(9): 13006–13022, https://doi.org/10.18632/aging.202981.
- Maute R.L., Gordon S.R., Mayer A.T., McCracken M.N., Natarajan A., Ring N.G., Kimura R., Tsai J.M., Manglik A., Kruse A.C., Gambhir S.S., Weissman I.L., Ring A.M. Engineering high-affinity PD-1 variants for optimized immunotherapy and immuno-PET imaging. Proc Natl Acad Sci U S A 2015; 112(47): E6506–E6514, https://doi.org/10.1073/pnas.1519623112.
- Mayer A.T., Natarajan A., Gordon S.R., Maute R.L., McCracken M.N., Ring A.M., Weissman I.L., Gambhir S.S. Practical immuno-PET radiotracer design considerations for human immune checkpoint imaging. J Nucl Med 2017; 58(4): 538–546, https://doi.org/10.2967/jnumed.116.177659.
- Natarajan A., Patel C.B., Ramakrishnan S., Panesar P.S., Long S.R., Gambhir S.S. A novel engineered small protein for positron emission tomography imaging of human programmed death ligand-1: validation in mouse models and human cancer tissues. Clin Cancer Res 2019; 25(6): 1774–1785, https://doi.org/10.1158/1078-0432.CCR-18-1871.
- Li C.W., Lim S.O., Chung E.M., Kim Y.S., Park A.H., Yao J., Cha J.H., Xia W., Chan L.C., Kim T., Chang S.S., Lee H.H., Chou C.K., Liu Y.L., Yeh H.C., Perillo E.P., Dunn A.K., Kuo C.W., Khoo K.H., Hsu J.L., Wu Y., Hsu J.M., Yamaguchi H., Huang T.H., Sahin A.A., Hortobagyi G.N., Yoo S.S., Hung M.C. Eradication of triple-negative breast cancer cells by targeting glycosylated PD-L1. Cancer Cell 2018; 33(2): 187–201.e10, https://doi.org/10.1016/j.ccell.2018.01.009.
- Du K., Huang H. Development of anti-PD-L1 antibody based on structure prediction of AlphaFold2. Front Immunol 2023; 14: 1275999, https://doi.org/10.3389/fimmu.2023.1275999.
- Sullivan K.M.C., Vilalta M., Ertl L.S., Wang Y., Dunlap C., Ebsworth K., Zhao B.N., Li S., Zeng Y., Miao Z., Fan P., Mali V., Lange C., McMurtrie D., Yang J., Lui R., Scamp R., Chhina V., Kumamoto A., Yau S., Dang T., Easterday A., Liu S., Miao S., Charo I., Schall T.J., Zhang P. CCX559 is a potent, orally-administered small molecule PD-L1 inhibitor that induces anti-tumor immunity. PLoS One 2023; 18(6): e0286724, https://doi.org/10.1371/journal.pone.0286724.
- Ertveldt T., De Beck L., De Ridder K., Locy H., de Mey W., Goyvaerts C., Lecocq Q., Ceuppens H., De Vlaeminck Y., Awad R.M., Keyaerts M., Devoogdt N., D’Huyvetter M., Breckpot K., Krasniqi A. Targeted radionuclide therapy with low and high-dose lutetium-177-labeled single domain antibodies induces distinct immune signatures in a mouse melanoma model. Mol Cancer Ther 2022; 21(7): 1136–1148, https://doi.org/10.1158/1535-7163.MCT-21-0791.
- Zhao H., Wang C., Yang Y., Sun Y., Wei W., Wang C., Wan L., Zhu C., Li L., Huang G., Liu J. ImmunoPET imaging of human CD8+ T cells with novel 68Ga-labeled nanobody companion diagnostic agents. J Nanobiotechnology 2021; 19(1): 42, https://doi.org/10.1186/s12951-021-00785-9.
- Nilofar Danishmalik S., Lee S.H., Sin J.I. Tumor regression is mediated via the induction of HER263-71- specific CD8+ CTL activity in a 4T1.2/HER2 tumor model: no involvement of CD80 in tumor control. Oncotarget 2017; 8(16): 26771–26788, https://doi.org/10.18632/oncotarget.15816.
- Penichet M.L., Challita P.M., Shin S.U., Sampogna S.L., Rosenblatt J.D., Morrison S.L. In vivo properties of three human HER2/neu-expressing murine cell lines in immunocompetent mice. Lab Anim Sci 1999; 49(2): 179–188.
- Shanghai Model Organisms Center, Inc. (SMOC): website. URL: https://www.modelorg.us/Humanized-cells.html.
- Biocytogen: website. URL: https://biocytogen.com/products/humanized-tumor-cell-lines/.
- GenOway: website. URL: https://www.genoway.com/catalog/cells/cl/overview.htm.
- Zheng Y., Fang Y.C., Li J. PD-L1 expression levels on tumor cells affect their immunosuppressive activity. Oncol Lett 2019; 18(5): 5399–5407, https://doi.org/10.3892/ol.2019.10903.
- Chen S., Crabill G.A., Pritchard T.S., McMiller T.L., Wei P., Pardoll D.M., Pan F., Topalian S.L. Mechanisms regulating PD-L1 expression on tumor and immune cells. J Immunother Cancer 2019; 7(1): 305, https://doi.org/10.1186/s40425-019-0770-2.
- Parvez A., Choudhary F., Mudgal P., Khan R., Qureshi K.A., Farooqi H., Aspatwar A. PD-1 and PD-L1: architects of immune symphony and immunotherapy breakthroughs in cancer treatment. Front Immunol 2023; 14: 1296341, https://doi.org/10.3389/fimmu.2023.1296341.
- Ilie M., Long-Mira E., Bence C., Butori C., Lassalle S., Bouhlel L., Fazzalari L., Zahaf K., Lalvée S., Washetine K., Mouroux J., Vénissac N., Poudenx M., Otto J., Sabourin J.C., Marquette C.H., Hofman V., Hofman P. Comparative study of the PD-L1 status between surgically resected specimens and matched biopsies of NSCLC patients reveal major discordances: a potential issue for anti-PD-L1 therapeutic strategies. Ann Oncol 2016; 27(1): 147–153, https://doi.org/10.1093/annonc/mdv489.
- McLaughlin J., Han G., Schalper K.A., Carvajal-Hausdorf D., Pelekanou V., Rehman J., Velcheti V., Herbst R., LoRusso P., Rimm D.L. Quantitative assessment of the heterogeneity of PD-L1 expression in non-small-cell lung cancer. JAMA Oncol 2016; 2(1): 46–54, https://doi.org/10.1001/jamaoncol.2015.3638.
- Radaram B., Glazer S.E., Yang P., Li C.W., Hung M.C., Gammon S.T., Alauddin M., Piwnica-Worms D. Evaluation of 89Zr-labeled anti-PD-L1 monoclonal antibodies using DFO and novel HOPO analogues as chelating agents for immuno-PET. ACS Omega 2023; 8(19): 17181–17194, https://doi.org/10.1021/acsomega.3c01547.
- Jagoda E.M., Vasalatiy O., Basuli F., Opina A.C.L., Williams M.R., Wong K., Lane K.C., Adler S., Ton A.T., Szajek L.P., Xu B., Butcher D., Edmondson E.F., Swenson R.E., Greiner J., Gulley J., Eary J., Choyke P.L. Immuno-PET imaging of the programmed cell death-1 ligand (PD-L1) using a zirconium-89 labeled therapeutic antibody, avelumab. Mol Imaging 2019; 18: 1536012119829986, https://doi.org/10.1177/1536012119829986.
- Bensch F., van der Veen E.L., Lub-de Hooge M.N., Jorritsma-Smit A., Boellaard R., Kok I.C., Oosting S.F., Schröder C.P., Hiltermann T.J.N., van der Wekken A.J., Groen H.J.M., Kwee T.C., Elias S.G., Gietema J.A., Bohorquez S.S., de Crespigny A., Williams S.P., Mancao C., Brouwers A.H., Fine B.M., de Vries E.G.E. 89Zr-atezolizumab imaging as a non-invasive approach to assess clinical response to PD-L1 blockade in cancer. Nat Med 2018; 24(12): 1852–1858, https://doi.org/10.1038/s41591-018-0255-8.
- Broos K., Lecocq Q., Xavier C., Bridoux J., Nguyen T.T., Corthals J., Schoonooghe S., Lion E., Raes G., Keyaerts M., Devoogdt N., Breckpot K. Evaluating a single domain antibody targeting human PD-L1 as a nuclear imaging and therapeutic agent. Cancers (Basel) 2019; 11(6): 872, https://doi.org/10.3390/cancers11060872.
- Christensen C., Kristensen L.K., Alfsen M.Z., Nielsen C.H., Kjaer A. Quantitative PET imaging of PD-L1 expression in xenograft and syngeneic tumour models using a site-specifically labelled PD-L1 antibody. Eur J Nucl Med Mol Imaging 2020; 47(5): 1302–1313, https://doi.org/10.1007/s00259-019-04646-4.
- Zhang Y., Cao M., Wu Y., Malih S., Xu D., Yang E., Younis M.H., Lin W., Zhao H., Wang C., Liu Q., Engle J.W., Rasaee M.J., Guan Y., Huang G., Liu J., Cai W., Xie F., Wei W. Preclinical development of novel PD-L1 tracers and first-in-human study of [68Ga]Ga-NOTA-RW102 in patients with lung cancers. J Immunother Cancer 2024; 12(4): e008794, https://doi.org/10.1136/jitc-2024-008794.
- Bamminger K., Pichler V., Vraka C., Limberger T., Moneva B., Pallitsch K., Lieder B., Zacher A.S., Ponti S., Benčurová K., Yang J., Högler S., Kodajova P., Kenner L., Hacker M., Wadsak W. Development and in vivo evaluation of small-molecule ligands for positron emission tomography of immune checkpoint modulation targeting programmed cell death 1 ligand 1. J Med Chem 2024; 67(5): 4036–4062, https://doi.org/10.1021/acs.jmedchem.3c02342.
- Luna-Gutiérrez M., Cruz-Nova P., Jiménez-Mancilla N., Oros-Pantoja R., Lara-Almazán N., Santos-Cuevas C., Azorín-Vega E., Ocampo-García B., Ferro-Flores G. Synthesis and evaluation of 177Lu-DOTA-PD-L1-i and 225Ac-HEHA-PD-L1-i as potential radiopharmaceuticals for tumor microenvironment-targeted radiotherapy. Int J Mol Sci 2023; 24(15): 12382, https://doi.org/10.3390/ijms241512382.
- He H., Qi X., Fu H., Xu J., Zheng Q., Chen L., Zhang Y., Hua H., Xu W., Xu Z., Chen X., You Q., Lin J., Huang G., Mao Y., Yu C. Imaging diagnosis and efficacy monitoring by [89Zr]Zr-DFO-KN035 immunoPET in patients with PD-L1-positive solid malignancies. Theranostics 2024; 14(1): 392–405, https://doi.org/10.7150/thno.87243.
- Heskamp S., Hobo W., Molkenboer-Kuenen J.D., Olive D., Oyen W.J., Dolstra H., Boerman O.C. Noninvasive imaging of tumor PD-L1 expression using radiolabeled anti-PD-L1 antibodies. Cancer Res 2015; 75(14): 2928–2936, https://doi.org/10.1158/0008-5472.CAN-14-3477.
- Masopust D., Sivula C.P., Jameson S.C. Of mice, dirty mice, and men: using mice to understand human immunology. J Immunol 2017; 199(2): 383–388, https://doi.org/10.4049/jimmunol.1700453.
- Beura L.K., Hamilton S.E., Bi K., Schenkel J.M., Odumade O.A., Casey K.A., Thompson E.A., Fraser K.A., Rosato P.C., Filali-Mouhim A., Sekaly R.P., Jenkins M.K., Vezys V., Haining W.N., Jameson S.C., Masopust D. Normalizing the environment recapitulates adult human immune traits in laboratory mice. Nature 2016; 532(7600): 512–516, https://doi.org/10.1038/nature17655.
- Lemma E.Y., Letian A., Altorki N.K., McGraw T.E. Regulation of PD-L1 trafficking from synthesis to degradation. Cancer Immunol Res 2023; 11(7): 866–874, https://doi.org/10.1158/2326-6066.CIR-22-0953.










