Assessment of Cytotoxic Effect Mechanisms of Gas-Discharge Plasma Radiation
The aim of the investigation was to assess the mechanisms of cytotoxic effect of gas-discharge plasma radiation on lymphosarcoma and breast cancer cells.
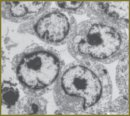
Materials and Methods. The experiment was carried out on the strains of rat lymphosarcoma (LSR) and breast cancer (RMK1) cells. 4 ml of cell suspension at (4–6)·106/ml concentration was exposed to gas-discharge plasma radiation in various time modes. Plasma radiation was generated by impulse device with the following set characteristics: burst time — 100 µs, voltage — 11 kV, energy per pulse — 5.9·10-2 J, pulse frequency — 10 Hz. Cytotoxic effect of gas-discharge radiation was assessed using fluorescent dye Hoechst (Sigma ALDRICH, USA), Propidium iodide (Sigma ALDRICH, USA) and МТТ-test. Structural changes in cells were studied by electron microscopy. Cytoplasmic membrane condition was assessed by microviscosity change using a hydrophobic fluorescent probe pyrene (Sigma ALDRICH, USA). The level of oxidative processes was determined by fluorescence of bityrosine, tryptophan, glycated proteins and lipid peroxidation processes. The state of coenzymes was estimated by NAD(P)+/NAD(P)Н+Н+ and FAD+/FADН2. DNA cell damage degree was assessed by DNA-comet assay.
Results. 600-second radiation exposed to LSR and RMK1 cells was found to be a half-lethal dose. Such radiation causes significant changes in the structure of cytoplasmic and nuclear membranes, intracellular content, reduces microviscosity indices both in a lipid bilayer and in protein-lipid interaction area of LSR and RMK1 cells. Protein molecules of these cells undergo marked oxidative modification exposed to gas-discharge plasma radiation. No accumulation of lipid peroxidation products was recorded. The content of reduced NAD(P)Н+Н+ and oxidized FAD+ increases in the cells under study under plasma radiation. The number of cells with significantly damaged DNA increases up to 81% by 600th second of exposure. All changes were in direct relationship to time exposure duration.
- Kieft I.E., Kurdi M., Stoffels E. Reattachment and apoptosis after plasma-needle treatment of cultures cells. IEEE Trans Plasma Sci 2006; 34(4): 1331–1336, http://dx.doi.org/10.1109/TPS.2006.876511.
- Fridman G., Peddinghaus M., Ayan H., Balasubramanian M., Gutsol A., Brooks A.D., Fridman A., Friedman G. Blood coagulation and living tissue sterilization by floating-electrode dielectric barrier discharge in air. Plasma Chem Plasma Process 2006; 26(4): 425–442, http://dx.doi.org/10.1007/s11090-006-9024-4.
- Laroussi M. Low temperature plasmas for medicine? IEEE Trans Plasma Sci 2009; 37(6): 714–725, http://dx.doi.org/10.1109/TPS.2009.2017267.
- Fridman G., Shereshevsky A., Jost M.M., Brooks A.D., Fridman A., Gutsol A., Vasilets V., Friedman G. Floating electrode dielectric barrier discharge plasma in air promoting apoptotic behavior in melanoma skin cancer cell lines. Plasma Chem Plasma Process 2007; 27(2): 163–176.
- Kim D., Gweon B., Kim D. B., Choe W., Shin J.H. A feasibility study for the cancer therapy using cold plasma. In: 13th International Conference on Biomedical Engineering. Singapore; 2009. Р. 355–357, http://dx.doi.org/10.1007/978-3-540-92841-6_87.
- Kim G., Lee H., Shon C. The effect of a micro plasma on melanoma (G361) cancer cells. Korean Phys Soc 2009; 54: 628–632.
- Ivanova I.P., Trofimova S.V., Karpel Vel Leitner N., Аristova N.А., Arkhipova Е.V., Burkhina О.Е., Sysoeva V.А., Piskaryov I.M. Analiz aktivnykh produktov izlucheniya plazmy iskrovogo razryada, opredelyayushchikh biologicheskie effekty v kletkakh [The analysis of active products of spark discharge plasma radiation determining biological effects in tissues]. Sovrem Tehnol Med — Modern Technologies in Medicine 2012; 2: 20–30.
- Ichetkina A.A., Trofimova S.V., Kryazhev D.V., Ivanova I.P., Smirnov V.F. Vliyanie ul’trafioletovogo izlucheniya i izlucheniya plazmy impul’snogo iskrovogo razryada na zarodyshevye struktury i mitseliy mikromitsetov-destruktorov [The effect of ultraviolet radiation and pulse spark discharge plasma radiation on embryonal structures and mycelium of micromyces-destructors]. Vestnik Nizhegorodskogo gosudarstvennogo universiteta im. N.I. Lobachevskogo — Vestnik of Nizhny Novgorod State University named after N.I. Lobachevsky 2011; 2(2): 196–201.
- Ivanova I.P., Trofimova S.V., Piskaryov I.M., Burkhina О.Е., Sysoeva V.А., Karpel Vel Leitner N. Issledovanie mekhanizmov biotsidnogo deystviya izlucheniya plazmy iskrovogo razryada [The study of biocidal mechanisms of spark discharge plasma radiation]. Sovrem Tehnol Med — Modern Technologies in Medicine 2012; 3: 12–18.
- Chery E. Balkman, Tracy L. Gieger, Marsha M. Zgola, Lionel D. Lewis, Margaret C. McEntee. In vitro characterization of Docetaxel as a radiosensitizer in canine and feline cancer cell lines. Open Journal of Veterinary Medicine 2012; 2: 285–292, http://dx.doi.org/10.4236/ojvm.2012.24045.
- Zhang H.-T., Luo H., Wu J., Lan L.-B., Fan D.-H., Zhu K.-D., Chen X.-Y., Wen M., Liu H.-M. Galangin induces apoptosis of hepatocellular carcinoma cells via the mitochondrial pathway. World J Gastroenterol 2010; 16(27): 3377–3384, http://dx.doi.org/10.3748/wjg.v16.i27.3377.
- Sapozhnikov A.G., Dorosevich A.E. Gistologicheskaya i mikroskopicheskaya tekhnika [Histological and microscopical equipment]. Smolensk: SAU; 2000; 476 p.
- Lutsenko M.T., Ishutina N.A. Sposob otsenki mikrovyazkosti membran eritrotsitov putem vychisleniya koeffitsienta eksimerizatsii pirena KEKS u beremennykh, perenesshikh obostrenie gerpes-virusnoy infektsii v tret’em trimestre gestatsii, s uchetom opredeleniya protsentnogo soderzhaniya oleinovoy kisloty v membranakh eritrotsitov [The assessment method of red blood cell membrane microviscosity by calculating pyrene excimerization coefficient Cexc in pregnant women with recurrent herpes virus infection in the third trimester of gestation based on percentage test of oleic acid in red blood cell membranes]. Patent RF №2467334. МПК G01N33/53. 2012.
- Deryugina A.V., Koryagin A.S., Kopylova S.V., Talamanova M.N. Metody izucheniya stressovykh i adaptatsionnykh reaktsiy organizma po pokazatelyam sistemy krovi [The methods to study stress and adaptive responses of the body by blood system indices]. Nizhny Novgorod: Izdatel’stvo Nizhegorodskogo gosuniversiteta; 2010; 25 p.
- Wheatley R.A. Some recent trends in the analytical chemistry of lipid peroxidation. Тrends in Analytical Chemistry 2000; 19: 617–628.
- Ivanova I.P., Piskarev I.M., Trofimova S.V. Initial stage of lipid peroxidation with HO2 radicals. Kinetic study. American Journal of Physical Chemistry 2013; 2(2): 44–51, http://dx.doi.org/10.11648/j.ajpc.20130202.13.
- Zhang X., Dudek E.J., Liu B., Ding L., Fernandes A.F., Liang J.J., Horwitz J., Taylor A., Shang F. Degradation of C-terminal Truncated αA-crystallins by the ubiquitin-proteasome pathway. Invest Ophthalmol Vis Sci 2007; 48: 4200–4208, http://dx.doi.org/10.1167/iovs.07-0196.
- Dubinina E.E., Gavrovskaya S.V., Kuz’mich E.V., et al. Okislitel’naya modifikatsiya belkov: okislenie triptofana i obrazovanie v ochishchennykh belkakh s ispol’zovaniem sistemy Fentona [Oxidative protein modification: tryptophan oxidation and formation in purified proteins using Fenton reaction]. Biokhimiya — Biochemistry 2002; 67: 413–421.
- Muthenna P., Akileshwari C., Saraswat M., Reddy G.B. Inhibition of advanced glycation end-product formation on eye lens protein by rutin. British Journal of Nutrition 2012 Apr; 107(7): 941–949. Epub 2011 Aug 25, http://dx.doi.org/10.1017/S0007114511004077.
- Farabegoli G., Hellinga C., Heijnen J.J., van Loosdrecht M.C. Study on the use of NADH fluorescence measurements for monitoring waste water treatment systems. Water Res 2003 Jun; 37(11): 2732–2738.
- Zherdeva V.V., Savitskiy A.P. Primenenie lantanidnogo induktivno-rezonansnogo perenosa energii pri izuchenii biologicheskikh protsessov in vitro i in vivo [The use of lanthanide energy transfer by inductive resonance when studying biological processes in vitro and in vivo]. Uspekhi biologicheskoy khimii — Advance of Biological Chemistry 2012; 52: 315–362.
- Durnev A.D., Zhanataev A.K., Anisina E.A., Sidneva E.S., Nikitina V.A., Oganesyants L.A., Seredenin S.B., Bekish V.Ya., Chernukha I.M. Primenenie metoda shchelochnogo gel’-elektroforeza izolirovannykh kletok dlya otsenki genotoksicheskikh svoystv prirodnykh i sinteticheskikh soedineniy [The application of the test of alkaline gel-electrophoresis of isolated cells to assess genotoxic properties of natural and synthetic compounds]. Moscow; 2006; 28 p.
- Boldyrev A.A., Kyayvyaryaynen E.I., Ilyukha V.A. Biomembranologiya [Biomembranology]. Petrozavodsk: Kar NTs RAN; 2006; 226 p.
- Ivanova I.P., Trofimova S.V., Piskaryov I.M., Ichetkina A.A., Burkhina О.Е., Sysoeva V.А. Vliyanie izlucheniya plazmy iskrovogo razryada na modifikatsiyu belkov i lipidov [The influence of the spark discharge plasma radiation on protein’s and lipid’s modification]. Fundamentalnie issledovania — Fundamental Research 2013; 1(3): 525–575.
- Severin E.S. Biokhimiya [Biochemistry]. Moscow: GEOTAR-MED; 2004; 779 p.
- Xia W., Wang Z., Wang Q., Han J., Zhao C., Hong Y., Zeng L., Tang L., Ying W. Roles of NAD / NADH and NADP+ / NADPH in cell death. Current Pharmaceutical Design 2009; 15(1): 12–19, http://dx.doi.org/10.2174/138161209787185832.
- Men’shikova E.B., Zenkov N.K., Reutov V.P. Oksid azota i NO sintazy pri razlichnykh funktsional’nykh sostoyaniyakh [Nitric oxide and NO synthase in different functional conditions]. Biokhimiya — Biochemistry 2000; 65(4): 485–503.
- Choi B.-M., Рае H.-O., Jang S.I. Nitric oxide as a pro-apoptotic as well as anti-apoptotic modulator. Journal of Biochemistry and Molecular Biology 2002; 35(1): 116–126.
- Vanin A.F. Oksid azota v biomeditsinskikh issledovaniyakh [Nitric oxide in biomedical researches]. Vestnik RAMN — Herald of the Academy of Sciences 2000; 4: 3–5.
- Starodubtseva M.N. Peroksinitrit v fiziologii i patologii kletok krovi [Peroxynitrite in physiology and pathology of blood cells]. Moscow: Knizhnyy dom «LIBROKOM»; 2011; 200 p.
- Kwak J.Y., Han M.K., Choi K.S., Park I.H., Park S.Y., Sohn M.H., et al. Cytokines secreted by lymphokine-activated killer cells induce endogenous nitric oxide synthesis and apoptosis in DLD-1 colon cancer cells. Cell Immunol 2000; 203(2): 84–94.










