Prognostic Value of Neurotrophic Factors and Neuron Specific Elonase in Patients with Extracerebral Brain Tumors
The aim of the investigation was to study the content of brain-derived neurotrophic factor (BDNF), glial-derived neurotrophic factor (GDNF) and neuron specific enolase (NSE) in patients with mature brain tumors before and after surgery and assess their prognostic value.
Materials and Methods. The study involved 39 women. Group 1 consisted of 16 virtually healthy subjects, group 2 — 23 patients with mature brain tumors, among them there were 10 patients with pituitary adenoma and 13 — with meningiomas. The examination of the patients included clinical and neurological examination, determination of BDNF, GDNF (R&D Systems, USA) and NSE (Vector Best, Russia) content in blood plasma using enzyme immunoassay (EIA) performed 5 days before the surgery, 5 days and 6 months after extracerebral oncotomy. There were also carried out pathomorphological study of postoperative material, neuro-imaging study.
Results. Mean BDNF level in blood plasma of patients was significantly higher than in healthy subjects. The comparative assessment of the content of neurotrophic factors in groups of patients with various tumor types showed that before surgical treatment there was the tendency for higher mean GDNF level in patients with meningiomas compared to those with pituitary adenomas, though the difference was not significant. The patients with the subsequent (6 months after surgical removal of mature brain tumor) recurrence were found to have higher
BDNF concentrations in blood plasma 5 days after surgery than the patients who had no continued tumor growth in first 6 months after the operation. The patients with continued tumor growth appeared to have imbalance in the dynamics of postoperative BDNF and GDNF levels.
Conclusion. BDNF concentration increase in blood plasma in the nearest time after surgical removal of a mature extracerebral brain tumor has unfavorable prognostic value in relation to the high probability of tumor recurrence in the subsequent 6 months.
Among neoplasms of the central nervous system (CNS) intracerebral (including neuroepithelial tumors), extracerebral (tumors of the brain membranes) tumors, and tumors of the Turkish saddle area (pituitary adenoma) are usually distinguished.
The meningiomas, pertaining to the extracerebral neoplasms, make 14–19% of the brain tumors (BT) [1]. They grow slowly, develop from the arachnoid granulations of the brain dura mater and are isolated from the brain. A five-year survival of patients with this type of BT makes 91%, recurrence rate during 10 years is, on the average, 20%, however, these percentages are defined by the completeness of tumor resection and specificity of neoplasm structure [1].
Pituitary adenomas are benign tumors of glandular epithelium of the hypophysis anterior lobe (adenohypophysis) and make up 6–18% of all intracranial tumors, 75% of adenomas show hormonal activity [1].
Though there exist a broad scope of neurovisualization methods necessary for early diagnosis and monitoring of the performed operative treatment, prognostic criteria for estimating the influence of extracerebral tumor growth on the brain tissue as a whole, and also for defining reparative processes in the brain after removal of the mass lesion, are poorly studied. Therefore, laboratory diagnosis, including identification of neuron-specific proteins — biologically active molecules, specific to the nervous tissue, and supporting normal functioning of the nervous system, is of great interest. Some of these proteins are markers of nervous system destruction (for example, neuron-specific enolase), others promote reparative processes. The latter include neurotrophic factors, which not only actively participate in the nervous system formation, but also help maintaining mature brain homeostasis. However, the role of neurotrophic factors in the compensatory and recovery processes in development and treatment of brain tumors is studied insufficiently.
Brain-derived neurotrophic factor (BDNF) and glial-derived neurotrophic factor (GDNF) are referred to neuron-specific proteins which are vital for brain functioning.
BDNF — is an important signal molecule participating in the regulation of neurogenesis, growth and survival of neurons in CNS [2]. Especially high level of BDNF expression in mature brain was found in the hippocampus and cerebral cortex areas [3], suggesting the most complete performance of the main functions of this protein in these departments of the CNS. The brain-derived neurotrophic factor plays an important role in ischemic and neurodegenerative diseases. It is shown, that BDNF takes part in the protective mechanisms of the nervous tissue in ischemic damage. At the same time, there are data on BDNF involvement in proliferation of neoplastic cells in meningiomas and pituitary adenomas [4, 5]. However, mechanisms of BDNF participation in progressing of tumor growth, and also in reparative processes after surgical removal of the tumor, are still not known. For clinical investigations of its role it is important, that BDNF plasma levels reflect the content of this factor in the CNS [6].
GDNF — a small protein belonging to the family of transforming growth factor beta, is secreted by glia cells (astrocytes, Schwann cells, etc.). This factor promotes preservation, proliferation and differentiation of various populations of cells of the central and peripheral nervous system [7]. The mRNA GDNF level quickly increases in case of nervous system damage and remains above normal values even in a week after trauma [8]. It is shown, that GDNF plays a role in the development of neurodegenerative diseases [9] and pituitary adenomas [10], however, there are no data on the content of GDNF in patients with brain meningiomas.
Neuron-specific enolase (NSE) is an isoform of enolase enzyme necessary for glycolysis. It is a specific serum marker of neuroendocrine tumors and nervous tissue destruction [11]. The NSE level is elevated in a stroke, brain injury, benign diseases of the brain, and is an unfavorable prognostic factor of neurologic deficiency [12].
Data on neutrophines involvement in the progression of neoplastic cells in extracellular BT and their participation in reparative processes after damage of CNS structures, make it reasonable to study the level ratios of neurotrophic factors and neuron-specific enolase in the course of mature extracellular neoplasm growth, and to estimate the possibilities of application of these data for predicting the recurrence of such tumors after their surgical removal.
The aim of the investigation was to study the content of brain-derived neurotrophic factor, glial-derived neurotrophic factor and neuron specific enolase in patients with mature extracerebral tumors before and after surgery, and to assess their prognostic value.
Materials and Methods. The study involved 39 women. Control group 1 consisted of 16 practically healthy subjects, aged between 46 and 59 years (mean age being 51.0±4.3 years), main group 2 consisted of 23 patients aged between 35 and 63 (mean age being 53.2±6.7 years) with mature extracerebral BT. Among them there were 10 patients with pituitary adenoma — subgroup 2a (9 — with prolactinoma and 1 — with somatotropinoma) — the mean age of which was 52.5±8.3 years, and 13 patients with brain meningiomas (the mean age of 53.7±5.5 years) — subgroup 2b.
Criteria of inclusion in the main group were: diagnosed mature BT, lack of marked mental disorders, and severe speech impairment complicating comprehension of verbal instructions and questions. Criteria of exclusion were: metastatic nature of brain damage, decompensating state of the patient (assessment according to the Karnovsky scale — 50 scores and lower), existence of diabetes mellitus, autoimmune diseases, heavy decompensated somatic illnesses, tumors of extracerebral localization, acute impairment of brain circulation, and acute myocardial infarction during the last 3 months in the history.
Examination of the patients with BT included clinical and neurological study, determination of BDNF, GDNF (R&D Systems, USA) and NSE (VectorBest, Russia) content in blood plasma using enzyme immunoassay (EIA) performed 5 days before surgery, 5 days and 6 months after extracerebral oncotomy. Pathomorphological study of the postoperative material, and neuro-imaging study were also carried out.
Neurovisualization investigation included multispiral computer tomography (Aquilion-64, Toshiba, Japan) carried out on the first day after surgery, and contrast-enhanced magnetic resonance tomography (GE Signa Infinity 1,5 T, USA) performed before and in 6 months after surgery.
In the control group neurotrophines and NSE content in the blood plasma was determined only once.
The study complies with the Declaration of Helsinki (the Declaration was passed in Helsinki, Finland, June, 1964, and revised in October, 2000, Edinburg, Scotland) and was performed following approval by the ethic committee of Nizhny Novgorod State Medical Academy. Written informed consent was obtained from every patient.
Experimental data were processed using a software package Statistica v7.0. Non-normal distributions of quantitative data were described by a median and interquartile range between 25th and 75th percentiles, i.e. the upper bound of the first and lower board of the forth quartile. Two samples were compared using Mann–Whitney U-test (for independent groups) for non-normal distributed data, and using Student’s t-test for independent groups when variables with normal distribution were analyzed. Parameter interrelationship was investigated by Spearman rank correlation coefficient. Differences were considered statistically significant at p<0.05
Results. The analysis of BDNF, GDNF and NSE levels in the studied groups (Table 1) revealed, that the average level of BDNF in patients’ blood plasma was statistically significantly higher, than in the control group (p=0.048). Statistically significant differences between mean concentrations of GDNF and NSE in plasma of healthy subjects and patients in different groups were not found (р=0.32 and 0.76, respectively).
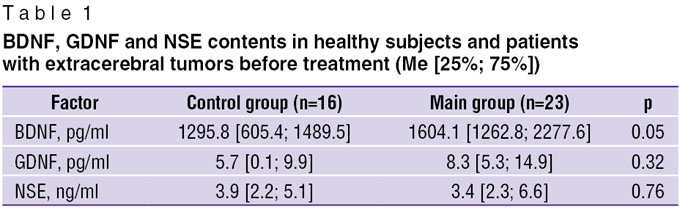 Table 1. BDNF, GDNF and NSE contents in healthy subjects and patients with extracerebral tumors before treatment (Me [25%; 75%]) Table 1. BDNF, GDNF and NSE contents in healthy subjects and patients with extracerebral tumors before treatment (Me [25%; 75%])
|
Comparative assessment of neurotrophic factor content in subgroups of patients with various types of tumors (meningioma and pituitary adenoma) showed, that before surgical treatment a tendency to a higher mean value of the GDNF level in patients with brain meningiomas was observed in comparison with patients with pituitary adenomas, though this difference was not statistically significant (Table 2). Subgroups 2а and 2b also did not have statistically significant differences in BDNF and NSE contents.
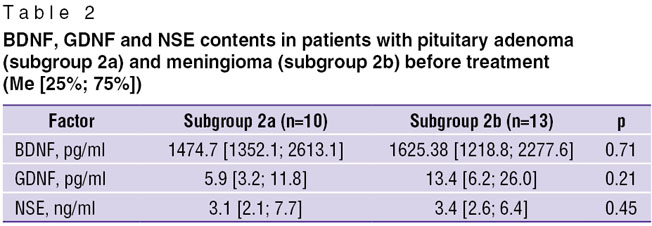 Table 2. BDNF, GDNF and NSE contents in patients with pituitary adenoma (subgroup 2a) and meningioma (subgroup 2b) before treatment (Me [25%; 75%]) Table 2. BDNF, GDNF and NSE contents in patients with pituitary adenoma (subgroup 2a) and meningioma (subgroup 2b) before treatment (Me [25%; 75%])
|
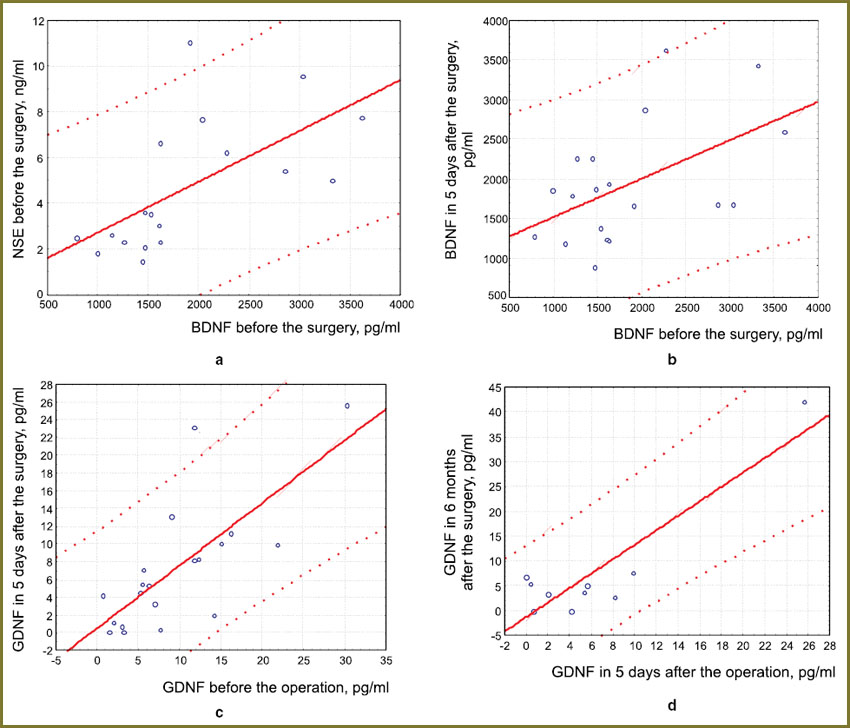
Statistically significant positive correlation was established between the contents of BDNF and NSE enzyme in blood plasma of the patients with extracerebral BT before surgery, r=0.63; p=0.0048 (Fig. 1, a). Correlation of BDNF factor levels before and after the operation was also found, r=0.52; p=0.023 (Fig. 1, b).
Along with this, correlation analysis in the group of patients revealed positive interrelations between GDNF factor levels, measured before and after surgical treatment, and also in 5 days and half a year after removal of the extracerebral tumor (r=0.74; p=0.0002 and r=0.91; p=0.0004 respectively) (Fig. 1, c, d). At the same time, correlation between NSE level and neurotrophic factors in the postoperative period and also between the NSE levels before neurosurgical treatment was not found.
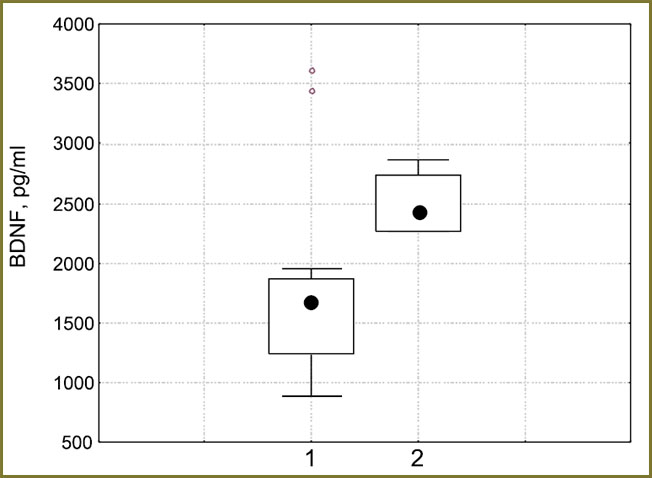
It is established, that the BDNF level in 5 days after the surgical removal of extracerebral tumor had a prognostic value of the tumor growth in postoperative period. Concentration of neurotrophine in the blood plasma of the patients with extracerebral BT in 5 days after surgery was higher in those patients in whom 6 months later continued tumor growth was found (2427.8 [2263.2; 2730.7] pg/ml), in comparison with patients without the continued mass lesion growth (1672.3 [1240.7; 1865.2] pg/ml), р=0.028 (Fig. 2). The fact that in all 4 patients (100%) with the revealed later-on-continued tumor growth the content of BDNF factor in 5 days after surgery exceeded the value of 2262 pg/ml, was worth paying attention. At the same time, in 13 of 15 (86.7%) patients, in whom the tumor did not recur during the following half a year, the content of BDNF in 5 days after the neurosurgical operation did not reach these values.
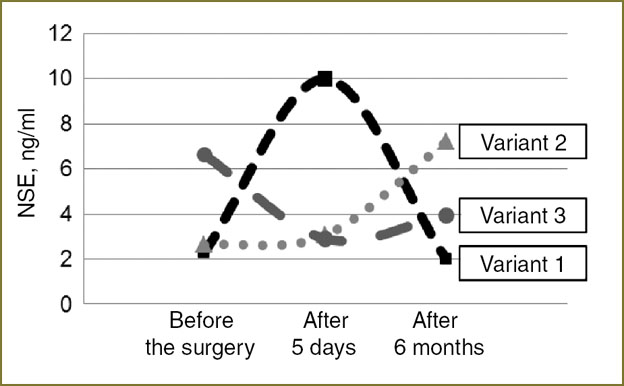
Three variants of BDNF concentration dynamics before surgical removal of mature BT were empirically determined. In the first variant, when BDNF level before the operation was initially low, i.e. less than 2037 pg/ml, there is a marked (1.5 times and more) rise of factor concentration in 5 days after surgery with a subsequent decrease (to the presurgical value) in half a year. The second variant is characterized by a reduction of BDNF factor in 5 days after the operation, but in half a year its level increases (two times or more). In the third variant there is a gradual growth of BDNF concentration in blood plasma in comparison with preoperative level both in 5 days after the operation and in half a year after the neurosurgical treatment (Fig. 3).
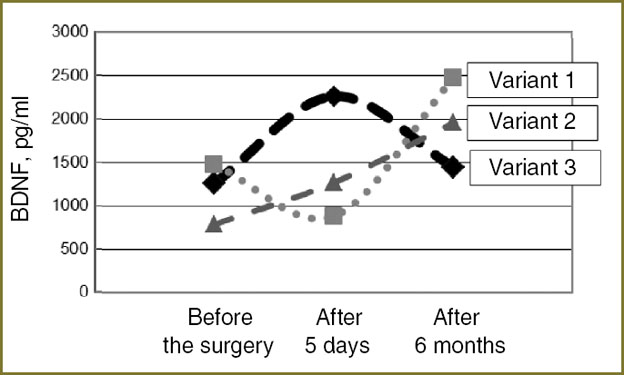 Fig. 3. Variants of BDNF dynamics in patients with mature tumors of the brain before, in 5 days, and in half a year after surgical treatment Fig. 3. Variants of BDNF dynamics in patients with mature tumors of the brain before, in 5 days, and in half a year after surgical treatment
|
Three variants of dynamic changes have been also determined according to plasma level of GDNF and NSE enzyme before and after surgical removal of mature BT. In the first variant with the average level of GDNF before the operation (less than 8.97 pg/ml) there is a decrease in its concentration in 5 days after the surgery (1.5 times and more) and a further decrease in half a year (Fig. 4). The second variant is characterized by weakening of GDNF concentration in 5 days after the operation, however, in half a year its level becomes twice as high as presurgical values or even more. In the third variant there is a gradual rise of GDNF level in blood plasma before and 5 days after surgical treatment, and its decrease in half a year after the operation.
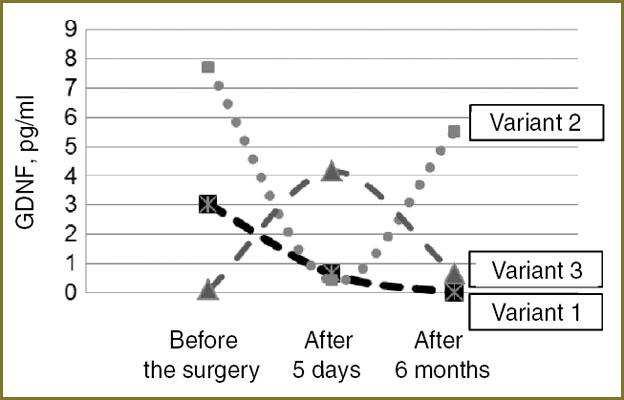 Fig. 4. Variants of GDNF dynamics in patients with mature tumors of the brain before, in 5 days, and in half a year after surgical treatment Fig. 4. Variants of GDNF dynamics in patients with mature tumors of the brain before, in 5 days, and in half a year after surgical treatment
|
In the first variant of NSE dynamics before and after BT oncotomy there was strengthening of its concentration in 5 days after the operation and its decrease in half a year after neurosurgical treatment (Fig. 5). In the second variant there is a gradual growth of NSE level in blood plasma before, in 5 days, and half a year after removal of extracerebral BT. The third variant is characterized by NSE level fall in 5 days after the operation, however, in half a year the level of the factor increases.
|
|
Studying various variants of neurotrophines concentration dynamics, it has been found, that the first variant of BDNF dynamics is typical for continued BT growth and has unfavorable prognostic value. Thus, it was in this subgroup of patients, consisting of 4 people (3 — with pituitary adenomas, 1 — with brain meningioma), that a reduction of GDNF level occurred in the post-operative period (Fig. 4, variant 1). It is also established, that the level of neuron-specific enolase does not correlate with neurotrophines concentration, found in patients with continued tumor growth.
Discussion. As reported by some authors [13, 14], the main functions, performed by BDNF, are connected with an interaction of this protein with TrkB receptor, necessary for triggering the signal mechanisms, which participate in various processes occurring in the cell. BDNF participates in neurogenesis stimulation, increases survival and stimulates growth of neurones of various phenotype and localization. Also, BDNF affects synoptic signal transmission, providing plasticity of nervous tissue, plays an important role in ischemic and neurodegenerative diseases. It was shown, that BDNF takes part in protective mechanisms of nervous tissue in ischemic damage. A positive feed-back has been recently found to exist between the BDNF level and function of microglia [15] activating in case of nervous tissue damage with subsequent formation of a neuroglial scar.
Our research showed, that in patients with extracerebral BT (meningiomas and pituitary adenomas), levels of the brain neurotrophic factor in blood plasma were higher before treatment, than in healthy subjects. We believe, that BDNF level increase can reflect a compensatory reaction of the nervous tissue, occurring in response to a gradual increase of tumor weight and compression of other brain tissues, and involving triggering of various intracellular signaling pathways. These pathways lead to the increase of gene expression responsible for cell survival, neurogenesis, growth of axons. Besides, average group concentrations of GDNF and NSE in blood plasma of patients and healthy objects did not differ. This fact indicates, that tumor growth does not influence the levels of these factors.
The GDNF functions are less studied. Concentration of the factor is high in the developing CNS. Knockout mice (with a switched-off gene, which is responsible for GDNF production) die at birth. In the adult state, partial knockout of GDNF gene or its receptor causes fast degradation of dopaminergic system and motion impairment [16, 17]. It was shown [8], that GDNF, when bounded with the family of GFR receptors, triggers a cascade of intracellular signaling pathways, activating intracellular kinases of the MAP cascade, inhibits apoptosis and increases survival of neurons in unfavorable conditions [18].
The research showed, that before surgical treatment a tendency to a higher mean value of GDNF level in patients with brain meningiomas in comparison with patients with pituitary adenomas was observed. Description of this fact is not found in scientific publications and it needs further investigation. BDNF and NSE levels were not found to have statistically significant differences in patients with pituitary adenomas and meningiomas. This shows, that the type of extracellular tumor does not affect the level of these factors, and concentration varies in rather wide limits.
Results of the research allowed us to reveal correlations of GDNF levels before surgery, after 5 days and in half a year after the removal of extracellular BT, and also a direct correlation between BDNF levels before and after surgery, between BDNF and NSE before the operation and lack of correlation of neurotrophic factors and NSE after oncotomy of extracerebral tumor. The observed close direct dependence between the NSE level (a neurone degradation marker) and BDNF before the operation can serve as an indirect confirmation of neuroprotective properties of BDNF.
In our opinion, such a newly discovered fact, that concentration of BDNF in blood plasma in 5 days after operation was higher in those patients, in which subsequently (in half a year after operative removal of a mature BT) the recurrence of the tumor was revealed, compared to the patients, in whom continued tumor growth did not occur in the next 6 months after the operation, deserves attention. These data suggest that BDNF level in the blood of patients after oncotomy may have prognostic value for defining the probability of further tumor recurrence.
The observed imbalance in dynamics of the postoperative BDNF and GDNF levels in patients with the continued neoplasm growth is most probably an indicator of disturbances in BDNF/GDNF-mediated compensatory mechanisms, activated in the postoperative period and directed to the recovery of both neuroglial element functions, damaged during the operation, and metabolism of all brain tissues.
Conclusion. BDNF level in blood plasma of patients was significantly higher than in healthy subjects. GDNF level in patients with meningiomas was higher compared to those with pituitary adenomas. BDNF concentration increase in blood plasma during several days immediately following surgical removal of a mature extracerebral brain tumor has unfavorable prognostic value in relation to a high probability of tumor recurrence in the subsequent 6 months.
Funding and Competing Interest. This study was supported by Russian Foundation for Basic Research grants No.13-04-01871, No.13-04-12067, No.14-04-31601. There is no topic specific conflict of interest related to the authors of this study.
References
- Krylov V.V. Lektsii po neyrokhirurgii [Lectures on Neurosurgery]. Moscow: KMK; 2008; 280 p.
- Fantacci C., Capozzi D., Ferrara P., Chiaretti A. Neuroprotective role of nerve growth factor in hypoxic-ischemic brain injury. Brain Sci 2013; 3(3): 1013–1022.
- Binder D.K., Scharfman H.E. Brain-derived neurotrophic factor. Growth Factors 2004 Sep; 22(3): 123–131, http://dx.doi.org/10.1080/08977190410001723308.
- Artico M., Bianchi E., Magliulo G., De Vincentiis M., De Santis E., Orlandi A., Santoro A., Pastore F.S., Giangaspero F., Caruso R., Re M., Fumagalli L. Neurotrophins, their receptors and KI-67 in human GH-secreting pituitary adenomas: an immunohistochemical analysis. Int J Immunopathol Pharmacol 2012 Jan–Mar; 25(1): 117–125.
- Artico M., Bronzetti E., Pompili E., Ionta B., Alicino V., D’Ambrosio A., Santoro A., Pastore F.S., Elenkov I., Fumagalli L. Immunohistochemical profile of neurotrophins in human cranial dura mater and meningiomas. Oncology Reports 2009 Jun; 21(6): 1373–1380, http://dx.doi.org/10.3892/or_00000363.
- Klein A.B., Williamson R., Santini M.A., Clemmensen C., Ettrup A., Rios M., Knudsen G.M., Aznar S. Blood BDNF concentrations reflect brain-tissue BDNF levels across species. Int J Neuropsychopharmacol 2011 Apr; 14(3): 347–353, http://dx.doi.org/10.1017/S1461145710000738.
- Yan Chen. Effects of glial cell line-derived neurotrophic factor (GDNF) on stem/progenitor cell proliferation and differentiation. Abstract of dissertation. UKnowledge; 2005; р. 233.
- Wang X., Liao Z.G., Liu M. GDNF expression following the severe brain injury in rats. Fa Yi Xue Za Zhi 2003; 19(1): 1–3.
- Airaksinen M.S., Saarma M. The GDNF family: signaling, biological functions and therapeutic value. Nat Rev Neurosci 2002 May; 3(5): 383–394.
- Japón M.A., Urbano A.G., Sáez C., Segura D.I., Cerro A.L., Diéguez C., Alvarez C.V. Glial-derived neurotropic factor and RET gene expression in normal human anterior pituitary cell types and in pituitary tumors. J Clin Endocrinol Metab 2002 Apr; 87(4): 1879–1884, http://dx.doi.org/10.1210/jcem.87.4.8383.
- Ahmed F., Gyorgy A., Kamnaksh A., Ling G., Tong L., Parks S., Agoston D. Time-dependent changes of protein biomarker levels in the cerebrospinal fluid after blast traumatic brain injury. Electrophoresis 2012 Dec; 33(24): 3705–3711, http://dx.doi.org/10.1002/elps.201200299.
- Singh H.V., Pandey A., Shrivastava A.K., Raizada A., Singh S.K., Singh N. Prognostic value of neuron specific enolase and IL-10 in ischemic stroke and its correlation with degree of neurological deficit. Clin Chim Acta 2013 Apr 18; 419: 136–138, http://dx.doi.org/10.1016/j.cca.2013.02.014.
- Patapoutian A., Reichardt L.F. Trk receptors: mediators of neurotrophin action. Curr Opin Neurobiol 2001 Jun; 11(3): 272–280, http://dx.doi.org/10.1016/S0959-4388(00)00208-7.
- Sakharnova T.A., Vedunova M.V., Mukhina I.V. Brain-derived neurotrophic factor (BDNF) and its role in central nervous system functioning. Neirokhimiya 2012; 24(4): 269–277.
- Elkabes S., DiCicco-Bloom E.M., Black I.B. Brain microglia/macrophages express neurotrophins that selectively regulate microglial proliferation and function. J Neurosci 1996 Apr 15; 16(8): 2508–2521.
- Pascual A., Hidalgo-Figueroa M., Gómez-Díaz R., López-Barneo J. GDNF and protection of adult central catecholaminergic neurons. J Mol Endocrinol 2011 Jun 8; 46(3): R83–R92, http://dx.doi.org/10.1530/JME-10-0125.
- Saavedra A., Baltazar G., Duarte E.P. Driving GDNF expression: the green and the red traffic lights. Prog Neurobiol 2008 Nov; 86(3): 186–215, http://dx.doi.org/10.1016/j.pneurobio.2008.09.006.
- Airaksinen M.S., Saarma M. The GDNF family: signalling, biological functions and therapeutic value. Nat Rev Neurosci 2002 May; 3(5): 383–394.