The Effect of Gas-Discharge Plasma Radiation on Erythrocyte Protein Modification
The aim of the investigation was to estimate the effect of spark plasma radiation on oxidative protein modification in solutions and erythrocytes in experiments and in vitro.
Materials and Methods. Pulse spark discharge generating low-temperature plasma radiation was formed using an experimental device PILIMIN series IR-10 (Russia). The characteristics of a discharge were the following: capacity of pulse capacitor — 3.3 nF, ballast resistance — 10 MΩ, power supply voltage — 11 kV, pulse recurrence frequency — 10 Hz. Tryptophan, albumin, hemoglobin solutions, and erythrocyte suspensions of intact animals and animals with experimental sarcoma were used as research subjects. 4 ml samples were treated in sterile Petri plates. Structural state of tryptophan, albumin and hemoglobin molecules was assessed by UV absorption spectra. Oxidative protein damage degree in solutions and cells was estimated by bityrosine and tryptophan fluorescence.
Results. The increase of oxidative protein modification in solutions after spark discharge plasma radiation is due to the presence of complexes of tryptophan, albumin and hemoglobin molecules with nitro compounds, nitric radicals, hydroperoxyl radicals formed under discharge generation. Erythrocyte protein structures of animals with experimental sarcoma are characterized by more intense oxidative modification compared to erythrocytes of intact animals. Oxidative modification of erythrocyte proteins under plasma radiation to greater degree is due to the accumulation of bityrosine cross-links.
As established by the previous authors, plasma radiation generates active particles [1], capable to cause oxidative damage of cell lipids and proteins. It is well known, that lipid molecules participate in reactions with free radicals. However, it was experimentally shown that there is no intensification of processes of lipid peroxide oxidation under the action of plasma radiation in cholesterol, triglyceride, common lipid model solutions [2], as well as in prokaryotic and eukaryotic cells [3, 4]. Amino acids, being component parts of peptides and protein macromolecules, are also the targets for radical oxidation. Free radicals attack proteins over the whole length of a polypeptide chain, breaking not only primary, but also secondary, and tertiary structure of proteins leading to increased sensitivity to proteolysis, inactivation of enzymes, impairment of protein native conformation with formation of large protein conglomerates or molecule fragmentations [5, 6].
Thus, the structural and functional integrity of protein molecules is one of the major factors determining cell activity regulation and its viability. Since biocidal effect of plasma radiation has been established [3, 4], and mechanisms of protein oxidation are not still clear enough, to study the effect of spark discharge plasma radiation on monocomponent solutions of amino acids and proteins and on cell protein molecules is urgent.
The aim of the investigation was to estimate the effect of spark plasma radiation on oxidative protein modification in solutions and in erythrocytes in experiments and in vitro.
Materials and Methods. The low-temperature plasma radiation was generated in the process of spark discharge formation by experimental device PILIMIN IR-10. The device was developed in Skobeltsyn Institute of Nuclear Physics, Lomonosov Moscow State University (Russia) in 2011. The characteristics of the applied spark discharge were as follows: pulse duration — 100 µs, front duration — 50 ns, power supply voltage — 11 kV, capacitance of the pulse capacitor — 3.3 nF, ballast resistance — 10 MΩ, pulse energy — 5.9·102 J, pulse repetition rate — 10 Hz.
The experiment included two stages. At the first stage model solutions of D-trypthophan (Reanal, Hungary), bovine serum albumin (Biomed-Krakov, Poland), bovine hemoglobin (Koch-Light Laboratories Ltd., UK) were the objects of investigation. The linear dependence of fluorescence intensity on the substance concentration had been revealed by preliminary investigation. The concentrations used in the research (tryptophan — 0.05 mM, albumin — 0.005%, a hemoglobin — 0.00014%) enabled registration of the maximum fluorescence intensity and excluded fluorescence quenching by excessive substance concentration. Tested substances were diluted in Hank’s balanced salt solution; and 4 ml samples were exposed to spark discharge plasma radiation during 30, 60, 300, 600 and 1200 s in sterile Petri plates. UF-absorption spectra as well as bityrosine and tryptophan fluorescence were assessed for the studied solutions. The solutions which were not exposed to plasma radiation served as a control.
At the second stage of the experiment suspensions of erythrocytes of intact animals and animals with experimental sarcoma were studied. Heparinized blood was received by decapitation of animals under ether anesthesia. Erythrocytes were washed three times in Hank’s balanced salt solution (10 min of centrifugation at 3000 rpm), and erythrocyte suspension in Hank’s solution (1:800) was prepared for investigation. Cell suspensions (4 ml) in sterile Petri plates were exposed to plasma radiation for 30, 60, 300, 600 and 1200 s. For the assessment of oxidative modification of intracellular and extramembrane protein structures following plasma radiation, erythrocyte hemolysis was performed: 1.5 ml of erythrocyte suspension and 1.5 ml of distilled water were combined in cuvette and fluorescence of tryptophan and bityrosine in hemolysate was measured. Tryptophan and bityrosine fluorescence intensity was related to the amount of the total hemoglobin and expressed in the relative units per gram of protein. Concentration of the total hemoglobin in erythrocyte suspension was measured by hemoglobincyanid method using “Hemoglobin-agate” kit (Agate-Med, Russia). Erythrocyte suspension not exposed to plasma radiation served as a control.
All measurements were made on spectrofluorimeter Fluorat-02 PANORAMA (Russia).
Spectrophotometric measurements of the model solutions were carried out in the absorption range of 200–400 nm which is typical for plasma emission products and the majority of organic compounds.
Fluorescence intensity was studied at the exciting wavelengths of 270 to 360 nm and emission — at 425 nm for bityrosine; for tryptophan the wavelengths were 280 to 290 nm and emission — 350 nm [5].
Experimental data were processed using software package Excel and Statistica 6.0. Results are presented as M±m, where M is arithmetic mean, m — standard error of mean. Statistical significance of the mean differences was determined using Student criteria.
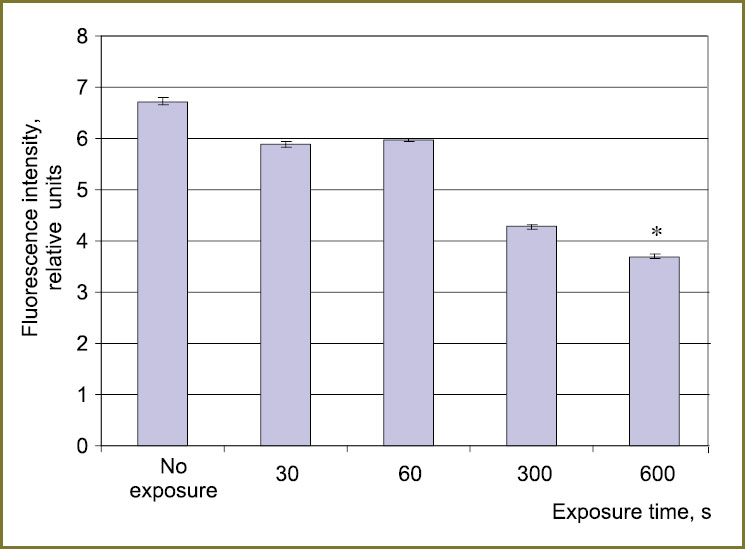
Results and Discussion. The analysis of oxidative modification of tryptophan in solution revealed the decrease of fluorescence intensity of the amino acid with the increase of plasma radiation exposure time (Fig. 1). This decrease may be the evidence of disturbances in tryptophan molecular structure [6]. Since the majority of organic compounds have absorption bands in UF area, the analysis of absorption spectra enables estimation of structural modifications of biomolecules in detail [7].
|
Fig. 1. Fluorescence intensity of the tryptophan solution (0.05 mM concentration) exposed to plasma radiation: * — statistical significance of values compared to the untreated series, p<0.05 |
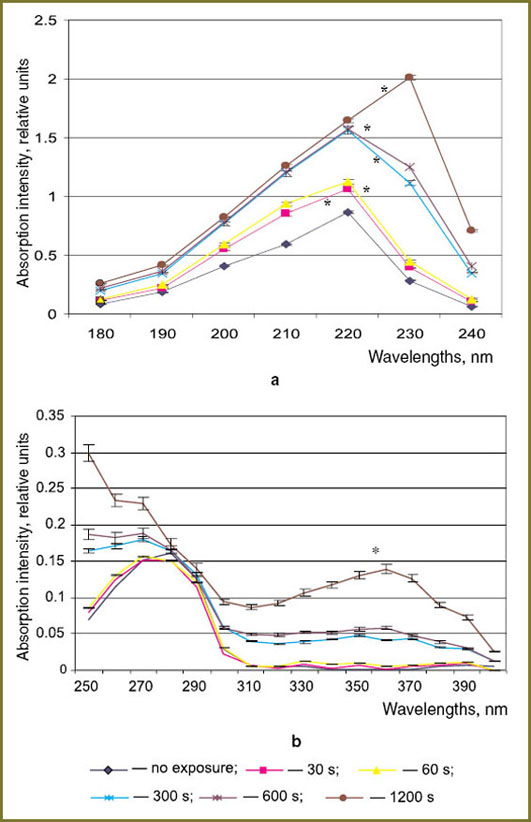
Analysis of absorption spectra of tryptophan solutions exposed to plasma radiation has revealed increase in absorption at 230 nm and 360 nm, as well as absorption band shift to shorter wavelengths from 280 to 260 nm (Fig. 2). According to the literature, n-π* junctions in C-H bonds in tryptophan pyrrole ring [8] and hydroperoxide radicals [9] are responsible for absorption in the area of 230 nm. It is also established that active particles, HO2 radicals in particular, are generated under the action of spark discharge [1, 10]. Probably, the increase of the peak value at 230 nm, being in direct relation to the time of exposure, is caused by binding HO2 radical to the tryptophan pyrrole ring. Shift of tryptophan absorption band may be the result of the molecule excitation by plasma radiation and electron density redistribution from nitrogen to oxygen that testifies to the decrease in the molecule stability, and could be caused, in particular, by formation of the «protein–protein» association and denaturation [11].
Absorption band at 360 nm corresponds to absorption of nitrogen electron pairs and –N=N– bond [12]. Generation of spark discharge plasma radiation is accompanied by accumulation of such active particles as NO–3, NH+4 and nitrosamines in gaseous and liquid phases [1, 10]. Registered increase in the absorption peak intensity at 360 nm is probably caused by formation of complexes of tryptophan benzene ring with nitro compounds.
The analysis of oxidative modification of albumin molecules exposed to plasma radiation revealed considerable 4-fold decrease in fluorescence intensity of tryptophan (Table 1). Tryptophan residues are part of the second domain of the first binding center of albumin globule [13] and, therefore, suppression of tryptophan fluorescence after plasma radiation exposure may indicate to the structural changes both in amino acids, and in the whole albumin molecule.
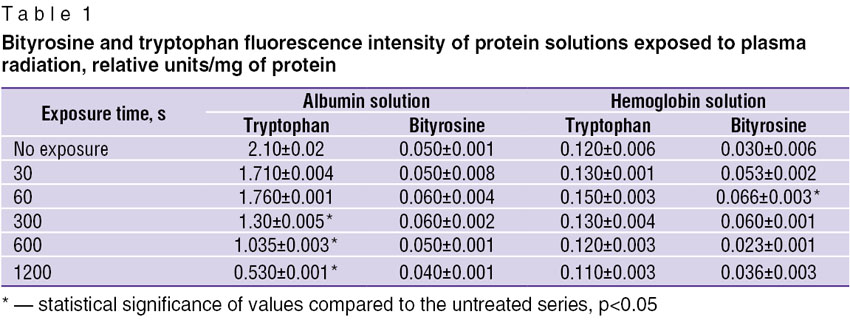
|
Table 1. Bityrosine and tryptophan fluorescence intensity of protein solutions exposed to plasma radiation, relative units/mg of protein |
The analysis of modification of hemoglobin exposed to plasma radiation for 60 s revealed statistically significant accumulation of bityrosine cross-links and fluorescence intensity decrease with the increased exposure time. As it is known that tyrosine fluoresces intensively during protein denaturation [14], it may be assumed that plasma radiation has a destructive effect on hemoglobin molecule.
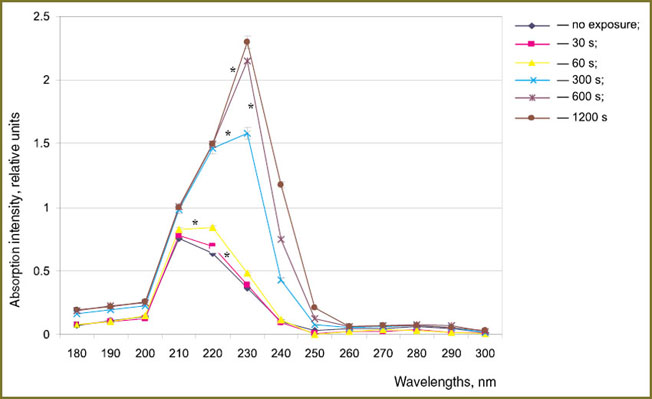
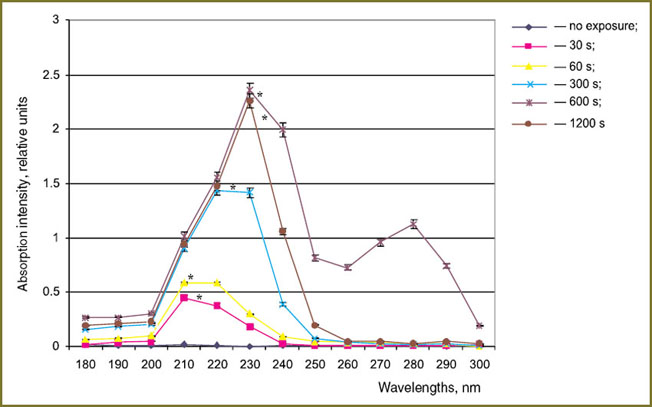
For studying the processes of proteins modification under gas-discharge plasma radiation in greater detail, spectral analysis of albumin and hemoglobin solutions was carried out. Absorption maxima at 210, 230, 280 nm both for albumin and hemoglobin exposed to plasma radiation (Fig. 3, 4) were revealed. It is known, that di-derivatives of SH groups are characterized by an absorption maximum at 210 nanometers [15]. One of the possible mechanisms of oxidative modification of proteins exposed to plasma radiation is cysteine SH groups oxidation. The absorption maximum near 230 m is probably caused by accumulation of peroxide groups in the protein molecules. According to the data obtained [16] nitro compounds (R-N-O2, O-NO2, N-NO2) and nitro groups associates have absorption band near 280 nm. Formation of R-N-O2 nitro compounds in the liquid phase during discharge generation is proved in the work [1]. Therefore, structural modification of albumin and hemoglobin intensifies with the increase of the exposure time due to formation of complexes with nitro particles. However for hemoglobin solution the maximum accumulation of nitro compounds is registered within the time period of 600 s and when the exposure time reaches 1200 s the absorption intensity peak decreases to the control values. The obtained results are in a good accordance with the findings of the work [10] in which decrease in nitrosamine concentration was found to occur at long exposure to plasma radiation.
The physical and chemical state and spectral properties of aromatic groups in cell proteins are often differ from those of amino acid and protein solutions. Investigating the effect of gas-discharge plasma radiation on erythrocytes with high percent of proteins in their structure, the level of cell protein modification may be judged (Table 2).
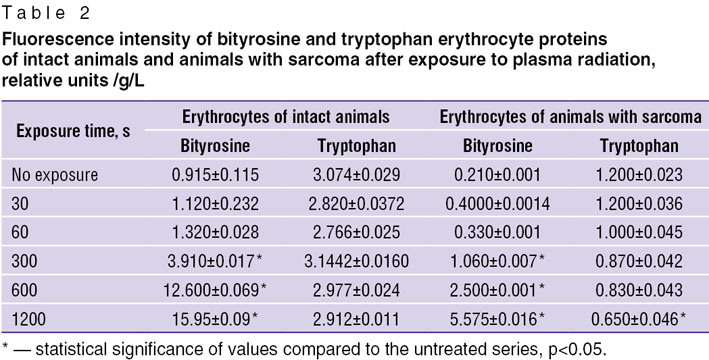
|
Table 2. Fluorescence intensity of bityrosine and tryptophan erythrocyte proteins of intact animals and animals with sarcoma after exposure to plasma radiation, relative units /g/L |
The analysis of fluorescence of erythrocytes tryptophan showed that the proteins in erythrocytes of animals with experimental sarcoma are in a more oxidized state that is in line with the data on the raised free radical activity in an organism with neoplastic process [17].
Exposure of erythrocytes of animals with sarcoma to plasma radiation resulted in 2-fold decrease of tryptophan fluorescence intensity. As for erythrocytes of intact animals, no changes were registered, which is presumably connected with the lower level of antioxidative activity in the blood of animals with the experimental tumor [18].
Comparison of bityrosine fluorescence level of non-exposed erythrocytes of intact animals and animals with experimental sarcoma showed that fluorescence intensity of erythrocytes of animals with a tumor is 4 times lower. Such results may speak of the cytoskeleton injury, and it is bityrosine cross-links that play an important role in its mechanical strength [19]. In the organisms with neoplastic process free radical activity is increased [20] that causes fragmentation of proteins and may be registered by the decrease in bityrosine cross-link fluorescence intensity.
The erythrocytes of intact animals, exposed to plasma radiation, showed a rise of bityrosine fluorescence intensity by 15 times, and in case of erythrocytes of animals with neoplastic process — by 26 times. In the independent researches of T. DiMarco, C. Giulivi, L.E. Muravleva [22, 23] it was shown that increase in bityrosine cross-link fluorescence intensity is most often caused by changes in protein tertiary and secondary structures. Thus, intensive formation and accumulation of bityrosine cross-links in erythrocyte proteins is taking place as a result of plasma radiation that may lead to modification of the tertiary and secondary protein structure.
Conclusion. Oxidative modification of tryptophan, albumin and hemoglobin molecules in solutions exposed to gas-discharge plasma radiation is caused by formation of complexes of these molecules with the following products of plasma radiation: nitro compounds, radicals of nitrogen, hydroperoxide radicals. Protein structures of erythrocytes of animals with experimental sarcoma become more oxidized in comparison with those of intact animals. Tyrosine molecules are less resistant to plasma radiation effect that causes oxidative modification of erythrocyte proteins of intact animals and animals with experimental sarcoma. Plasma radiation induces modification of tertiary and secondary structure of erythrocyte proteins, impairs cytoskeleton structure and functional activity of the whole cell.
Study Funding and Conflict of Interests. The study was not supported by any financial sources, and the authors have no conflict of interest to disclose.
References
- Ivanova I.P., Trofimova S.V., Karpel Vel Leitner N., Аristova N.А., Arkhipova Е.V., Burkhina О.Е., Sysoeva V.А., Piskaryov I.M. The analysis of active products of spark discharge plasma radiation determining biological effects in tissues. Sovremennye tehnologii v medicine 2012; 2: 20–30.
- Ivanova I.P., Trofimova S.V., Piskaryov I.M. The influence of the spark discharge plasma radiation on protein’s and lipid’s modification. Fundamentalnie issledovania 2013; 1(3): 572–575.
- Ivanova I.P., Trofimova S.V., Piskaryov I.M., Burkhina О.Е., Sysoeva V.А., Karpel Vel Leitner N. The study of biocidal mechanisms of spark discharge plasma radiation. Sovremennye tehnologii v medicine 2012; 3: 12–18.
- Ivanova I.P., Trofimova S.V., Vedunova М.V., Zhabereva А.S., Bugrova M.L., Piskaryov I.M., Karpel Vel Leitner N. Assessment of cytotoxic effect mechanisms of gas-discharge plasma radiation. Sovremennye tehnologii v medicine 2014; 6(1): 14–22.
- Dubinina E.E., Gavrovskaya S.V., Kuz’mich E.V., Leonova N.V., Morozova M.G., Kovrugina S.V., Smirnova T.A. Oxidative protein modification: tryptophan oxidation and bityrosine formation in purified proteins using Fenton system. Biokhimiya 2002; 67: 413–421.
- Hixon J., Reshetnyak Y.K. Algorithm for the analysis of tryptophan fluorescence spectra and their correlation with protein structural parameters. Algorithms 2009; 2: 1155–1176, http://dx.doi.org/10.3390/a2031155.
- Pentin Yu.A., Kuramshina G.M. Osnovy molekulyarnoy spektroskopii [Basics of molecular spectroscopy]. Moscow: Mir; 2008; 398 p.
- Nikitina L.E., Plemenkov V.V. Fizicheskie metody identifikatsii organicheskikh soedineniy [Physical methods of identification of organic compounds]. Kazan 2003; 92 p.
- Mel’nikov M.Ya. Photochemistry of peroxide radicals in solid phase and on an activated surface of solid bodies. Vestnik Moskovskogo universiteta. Khimiya 2001; 42(3): 188–193.
- Piskaryov I.M., Ivanova I.P., Trofimova S.V., Aristova N.A. Formation of active particles in spark discharge and their possible application. Khimiya vysokikh energiy 2012; 46(5): 406–411.
- El’yashevich M.A. Atomnaya i molekulyarnaya spektroskopiya [Atomic and molecular spectroscopy]. Moscow: Editorial URSS; 2001; 896 p.
- Anisimova N.A. Identifikatsiya organicheskikh soedineniy [The identification of organic compounds]. Gorno-Altaysk: RIO GAGU; 2009; 95 p.
- Quinlan G.J., Martin G.S., Evans T.W. Albumin: biochemical properties and therapeutic potential. Hepatology 2005; 41(6): 1211–1219.
- Kazakova V.V., Elkina N.M. Oxidative modifications of the changes of intramolecular hydrophobic property of hemoglobin A in human erythrocyte incubation in Fenton medium. Ukraїns’kiy bіokhіmіchniy zhurnal 2007; 79(4): 34–38.
- Karnaukhova L.I., Tupitsyn E.N. UF-spektroskopiya biologicheskikh makromolekul [UV spectroscopy of biological macromolecules]. Saratov: SGU; 2002.
- Vyaz'min S.Yu., Ryabukhin D.S., Vasil'ev A.V. Elektronnaya spektroskopiya organicheskikh soedineniy [Electronic spectroscopy of organic compounds]. Saint Petersburg: SPbGLTA; 2011.
- Kroemer G., Pouyssegur J. Tumor cell metabolism: cancer’s Achilles’ heel. Cancer Cell 2008 Jun; 13(6): 472–482, http://dx.doi.org/10.1016/j.ccr.2008.05.005.
- Dubinina E.E. Produkty metabolizma kisloroda v funktsional’noy aktivnosti kletok (zhizn’ i smert’, sozidanie i razrushenie). Fiziologicheskie i kliniko-biokhimicheskie aspekty [Oxygen metabolism products in cell functional activity (life and death, creativeness and destruction). Physiological, clinical and biochemical aspects]. Saint Petersburg: Meditsinskaya pressa; 2006; 400 p.
- Gubskiy Yu.I., Belenichev I.F., Pavlov S.V. Toxicological sequences of oxidative protein modification in various pathological conditions (Review). Sovremennye problemy toksikologii 2005; 3: 20–26.
- Men’shchikova E.B. Okislitel’nyy stress: patologicheskie sostoyaniya i zabolevaniya [Oxidative stress: pathological conditions and diseases]. Novosibirsk: ARTA; 2008; 284 p.
- Abalenikhina Y.V., Fomina M.A., Churilov G.I., Ivanycheva Y.N. Oxidative protein modification rats thymus under the influence of copper in ultrafine form. Fundamentalnie issledovania 2012; 11(6): 1315–1319.
- DiMarco T., Giulivi C. Current analytical methods for the detection of dityrosine, a biomarker of oxidative stress, in biological samples. Mass Spectrom Rev 2007; 26(1): 108–120, http://dx.doi.org/10.1002/mas.20109.
- Muravleva L.Ye., Molotov-Luchansky V.B., Klyuyev D.A., et al. Oxidative protein modification: problems and research prospects. Fundamentalnie issledovania 2010; 1: 74–78.