The Effect of Sterofundin and Cytoflavin on Morphological Changes in Myocardium in Acute Massive Blood Loss in Experiment
The aim of the investigation was to study in experiment the effect of antihypoxic drugs (Sterofundin and Cytoflavin) on morphology and morphometric indices of myocardium after acute massive blood loss.
Materials and Methods. The experiments were carried out on 54 Wistar male rats, weighing 230–250 g. Hemorrhagic shock was simulated by acute massive blood loss at the rate of 2 ml/min. Blood loss in an hour was followed by hypovolemia replacement within 60 min in the volume of 200% of lost mass: in control series — by Ringer`s solution, in the first trial series — by isotonic Sterofundin, in the second trial series — by isotonic Sterofundin and metabolic support by Cytoflavin. Blood reinfusion in the volume of 70% of blood loss was carried out. Morphological and morphometric myocardial changes were assessed 1 and 3 days after hemorrhagic shock.
Results. We have revealed cardioprotective effect of combined use of malate-containing blood substitute isotonic Sterofundin and Cytoflavin in infusion therapy of experimental hemorrhagic shock.
The problem of acute massive blood loss (AMBL) and hemorrhagic shock is still urgent because of a high mortality rate due to multi-organ failure [1–3].The most important pathogenetic factor of multi-organ failure in hemorrhagic shock is hypovolemia causing hypoperfusion and ischemic damage of organs and tissues including myocardium [4–6].
Infusion therapy is an integral part of AMBL treatment. New infusion preparations and their role in the correction of homeostasis impairment in an early post-hemorrhagic period need further studies [7].
The aim of the investigation was to study in experiment the effect of antihypoxic drugs (Sterofundin and Cytoflavin) on morphology and morphometric indices of myocardium after acute massive blood loss.
Materials and Methods. The experiments were carried out on 54 Wistar male rats, weighing 230–250 g. The work was carried out in full agreement with the ethical principles of European Convention for the protection of vertebrata used for experimental and other scientific purposes (the convention was passed in Strasburg, 18.03.1986, and adopted in Strasburg, 15.06.2006). AMBL was induced by Nembutal (25 mg/kg) using blood exfusion (blood volume 2.5 ml/100 g at the rate of 2 ml/min), 30% of total blood volume, from the caudal artery catheterized for direct arterial blood pressure measurement. In the period of hypovolemia, before blood replacement, MAP was recorded within 40–50 mm Hg with no pharmaceutical support. In an hour AMBL was followed by hypovolemia replacement by a chosen preparation in the volume of 200% of blood mass. Time of infusion preparation administration was 60 min. Infusion by crystalloid preparations was followed by blood reinfusion in the volume of 70% of blood loss. The blood of control experimental animals (n=18) was replaced by Ringer`s solution, the first series animals (n=18) — by isotonic Sterofundin (B. Braun, Germany), the second series animals (n=18) — by isotonic Sterofundin with metabolic support of Cytoflavin injected 1 h after blood replacement and reinfusion. The intensity of morphological changes was assessed 24 and 72 h after AMBL. Light-optical microscopy was carried out in histological myocardial preparations after their 72–96-hour fixation in 10% neutral formalin solution, dehydration in high-proof alcohol and paraffin embedding. Slices 7 µm thick were prepared on microtome Leica SM 2000R and hematoxylin and eosin stained. The histological preparations were examined, the images were recorded and morphological studies were carried out using microvisor Vizo-103 (Russia). The fields of vision on slices were chosen by the random numbers method (Avtandilov G.G., 1984). The following structures were studied: capillary diameter (µm), the number of functioning capillaries per 1 mm2 myocardial slice area, cardiomyocyte diameter (µm), area of cardiomyocyte nuclei (µm2).
The findings were statistically processed using Microsoft Excel and Statistica 6.0 by nonparametric criteria using Kruskal–Wallis test ANOVA.
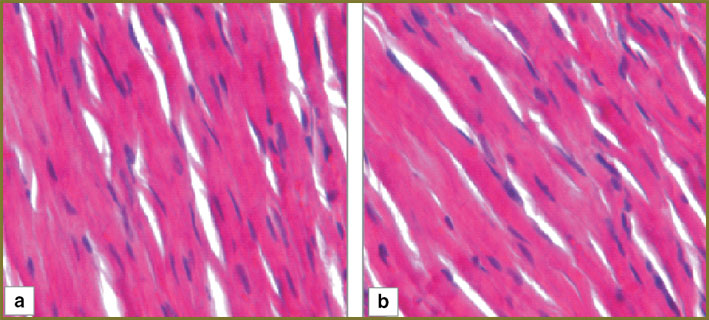
Results and Discussion. In the myocardium of the control series animals 1 day after hemorrhagic shock there was observed marked perivascular and pericellular edema, cardiomyocytes being arranged by parallel bundles with overcontraction and rupture areas (Fig. 1, а). One third of arterioles had mixed thrombi overlapping up to 1/2–2/3 of their lumen (Fig. 2, а).
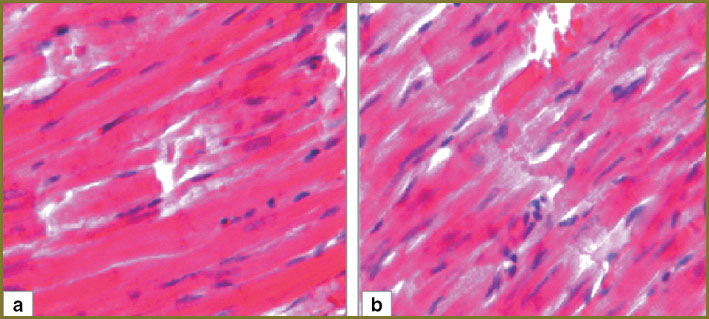 Fig. 1. Myocardial structure of the control series: а — day 1; b — day 3. Hematoxylin and eosin staining, ×250 Fig. 1. Myocardial structure of the control series: а — day 1; b — day 3. Hematoxylin and eosin staining, ×250
|
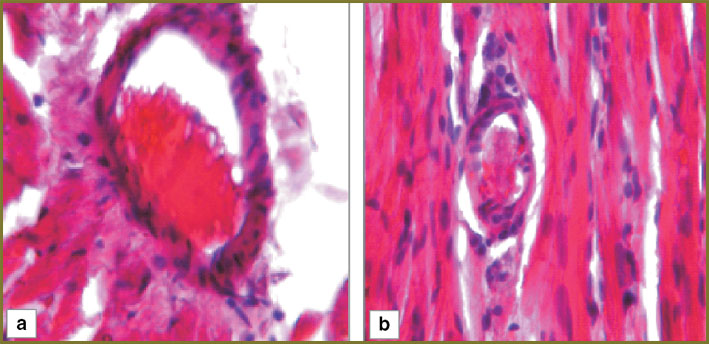 Fig. 2. Myocardial structure of the control series (arteriola): а — day 1; b — day 3. Hematoxylin and eosin staining, ×250 Fig. 2. Myocardial structure of the control series (arteriola): а — day 1; b — day 3. Hematoxylin and eosin staining, ×250
|
In the myocardium of the control series animals 3 days after hemorrhagic shock there persisted marked perivascular and pericellular edema, the number of overcontraction and muscular fiber rupture areas increasing (Fig. 1, b). Nearly half of myocardial arterioles had mixed thrombi overlapping up to 2/3–3/4 of their lumen (Fig. 2, b).
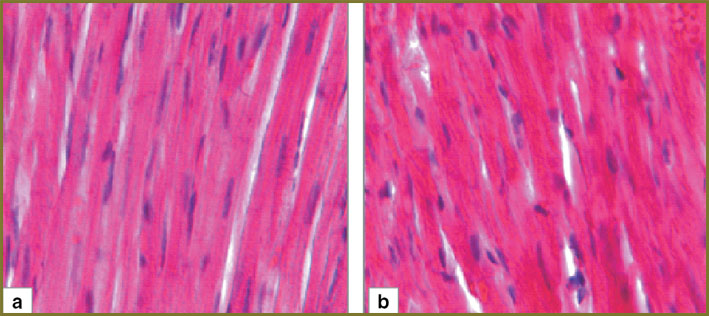
In the first experimental series animals, 24 h after hemorrhagic shock we determined in myocardium moderate perivascular and pericellular edema. Cardiomyocytes were parallel to each other, capillaries being observed between them. Lumen of the most capillaries had no formed elements. There were no overcontraction and cardiomyocyte rupture areas (Fig. 3, а). In myocardial arterioles there were seen loose lying erythrocytes and single sludges (Fig. 4, а).
|
|
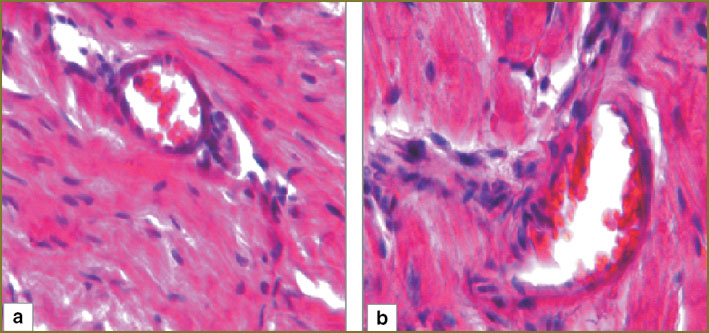 Fig. 4. Myocardial structure of the first experimental series (arteriole): а — day 1; b — day 3. Hematoxylin and eosin staining, ×250 Fig. 4. Myocardial structure of the first experimental series (arteriole): а — day 1; b — day 3. Hematoxylin and eosin staining, ×250
|
3 days after hemorrhagic shock in myocardium of the first experimental series moderate perivascular and pericellular edema persisted. Lumen of the most capillaries had no formed elements. The areas of overcontraction and cardiomyocyte rupture were not found (Fig. 3, b). 1/3 of myocardial arterioles had sludged erythrocytes (Fig. 4, b).
In the second experimental series animals, 1 day after hemorrhagic shock we determined in myocardium mild perivascular and pericellular edema, capillaries being equally spaced, parallel to cardiomyocytes. In myocardium there were no overcontraction and cardiomyocyte rupture areas (Fig. 5, а). Myocardial arterioles had no sludged erythrocytes, their lumen being free of formed elements (Fig. 6, а).
|
|
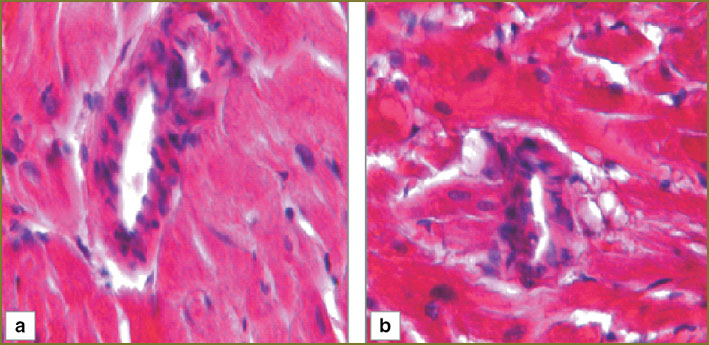 Fig. 6. Myocardial structure of the second experimental series (arteriole): а — day 1; b — day 3. Hematoxylin and eosin staining, ×250 Fig. 6. Myocardial structure of the second experimental series (arteriole): а — day 1; b — day 3. Hematoxylin and eosin staining, ×250
|
Histologic myocardial pattern of the second experimental series 3 days after hemorrhagic shock was similar to that after 1 day after hemorrhagic shock (Fig. 5, b and 6, b).
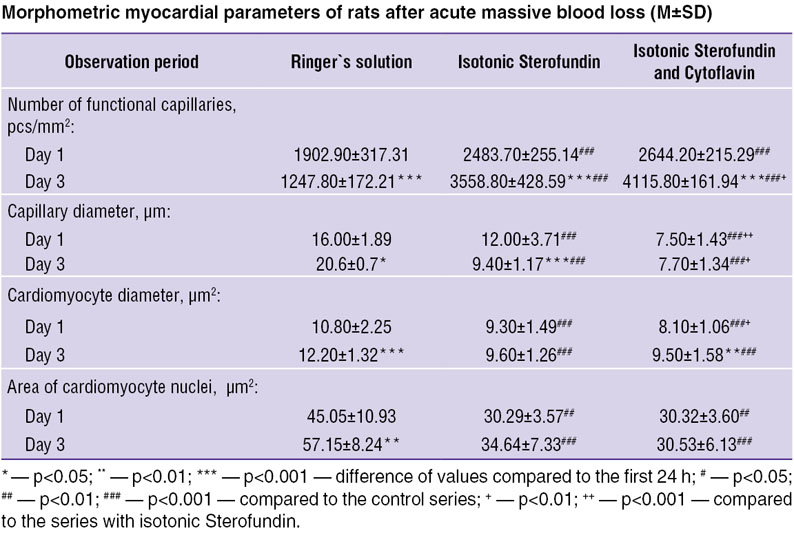
To objectivize the histological findings we studied myocardial structural elements morphometrically (See the Table).
|
|
The analysis of myocardial capillary diameter showed its significantly highest value in the control series, the value growing by the day 3 (22.33%). In the first experimental series this parameter decreased by 21.66% by the day 3, there was revealed a significant difference with the control series of experiments. In the animals taking additional Cytoflavin, the diameter of myocardial capillaries remained unchanged, significant difference being determined on day 1 and 3 of the experiment in the control and the first experimental series. The changes indicated a marked paresis of the capillary bed in the control series, in which Ringer`s solution had been used for the compensation for circulating blood volume. In the first experimental series 1 day after AMBL there was revealed paresis of capillaries with its resolution by the day 3. The second experimental series was characterized by a stable diameter of capillaries, there being no capillary paresis.
The dynamic study of the number of functioning (open) capillaries in the control series of experiments showed the lowest values decreasing by 34% by the day 3 compared to those observed in the experimental series. So, the growing number of functioning capillaries was 30.21 and 35.75% in the first and the second experimental series.
The analysis of cardiomyocyte diameter demonstrated that in all series there was unidirectional growth of this value with significantly lower results compared to the experimental series. Similar dynamics was found in the value of cardiomyocyte nuclear area.
Thus, the experimental animals of the control series had more evident edema of cardiomyocyte nuclei and cytoplasm.
The findings indicate that AMBL therapy consisting in the administration of isotonic Sterofundin and its combination with Cytoflavin prevents the formation of mixed thrombi in myocardial arterioles and reduces the number of sludges promoting the improved oxygen delivery to cardiomyocytes preventing irreversible changes in them (ruptures in overcontraction areas). In addition to the above mentioned positive effect on myocardial microvasculature, cytoprotective properties of the administered drugs appear in reduced nuclear and cytoplasmic edema of cardiomyocytes that is more likely to be due to their effect on the optimization of oxidative phosphorylation and “substrate hunger” correction in myocardial cells [8–14].
The constant growth of technogenic and natural disasters, terrorist attacks and armed conflicts is accompanied by a large number of injured and wounded with hypovolemia, which develops due to the decreased total blood volume and results in impaired circulatory dynamics, oxygen microcirculation and delivery with irreversible morphological damage leading to multi-organ failure [15, 16].
Blood loss is still the main cause of death in traumatic injuries, and for this reason hypovolemia should to be corrected immediately and to the full extent using infusion agents. The use of preparations containing substrate antihypoxic drugs enables to expand the opportunities of pathogenetically reasonable infusion therapy of hemorrhagic shock.
However, it must be admitted that current substrate antihypoxic agents added in polyionic solutions and solutions for parenteral feeding are considered only as a source of reserve buffer capacity. Much attention of foreign researchers is focused on the use of these medicinal agents to correct water-electrolytic and acid-base impairments [17–19].
Complex dynamics of body response on hemorrhagic shock, a wide spectrum of functional and metabolic systems involved in the process, these systems limiting the areas and mechanisms that make it multi-component, multi-organ and multi-stage, explain why despite a long history of shock study, currently, many aspects of the effect of substrate antihypoxic agents on myocardium in blood loss are still uninvestigated.
Significant energy imbalance occurs at early stages of hemorrhagic shock, and it is likely to have an impact on myocardial morphology and functions. However, in spite of the strong evidence of myocardial metabolic imbalance in post-hemorrhagic period, and a great number of studies devoted to the problem, no morphometric studies of myocardium in different forms of infusion therapy using substrate antihypoxic agents (Sterofundin, Cytoflavin) have been carried out [11–13, 20, 21].
In this study for the first time we showed by experiments an effective combined use of Sterofundin and Cytoflavin to prevent irreversible morphological changes in myocardium in hemorrhagic shock.
The gathered clinical experience showed high efficiency of cardioprotective techniques in cardiac surgery; however, very few studies have been carried out in hemorrhagic shock, in which cardioprotetion is important as well reducing the intensity of morphological manifestations of myocardial damage that enables to decrease lethality and reduce the extent of intensive therapy.
Conclusion. The use of isotonic Sterofundin solution and its combination with Cytoflavin in infusion therapy of experimental acute massive blood loss prevents the development of irreversible structural changes in myocardium in early period after blood loss.
Study Funding and Conflict of Interests. The study was not supported by any financial sources, and the authors have no conflict of interest to disclose.
References
- Gerasimov L.V., Karpun N.A., Pirozhkova O.S. Selected problems of pathogenesis and intensive therapy of major concomitant injury. Obshchaya reanimatologiya 2012; 8(4): 111–117.
- Spahn D.R., Cerny V., Coats T.J., et al. Management of bleeding following major trauma: a European Guidline. Crit Care 2007; 11(1): R17, http://dx.doi.org/10.1186/cc5686.
- Yang J.F., Sun L.M., Wang X.F., Dai N. Massive gastrointestinal bleeding from Meckel diverticulum with ectopic pancreatic tissue. Chin Med J (Engl) 2011 Feb; 124(4): 631–633.
- Schmid-Schonbein G.V., Delano F.A., Penn A.N., Kistler E. An elementary analysis of physiologic shock and multi-organ failure: the autodigestion hypothesis. Conf Proc IEEE Eng Med Biol Soc 2012 Aug; 2012: 3114–3115, http://dx.doi.org/10.1109/EMBC.2012.6346623.
- Al’es V.F., Stepanova N.A., Gol’dina O.A., et al. Pathophysiological mechanisms of oxygen delivery, consumption and extraction in critical conditions. Their intensive care methods. Vestnik intensivnoj terapii 1998; 2: 8–12.
- Mazurkevich G.S., Bagnenko S.F. Shok: teoriya, klinika i organizatsiya protivoshokovoy pomoshchi [Shock: theory, clinical picture and anti-shock aid organization]. Saint Petersburg: Politekhnika; 2004.
- Moroz V.V. Strategiya i taktika primeneniya antigipoksantov pri kriticheskikh sostoyaniyakh. V kn.: Fundamental’nye problemy reanimatologii (izbrannye lektsii i obzory). Trudy instituta obshchey reanimatologii RAMN [Strategy and tactics of using antihypoxic agents in critical conditions. In: Fundamental problems of emergency medicine (selective lectures and reviews). Proceedings of Research Institute of General Reanimatology of RAMS]. Vol. IV. Moscow; 2005; p. 210–220.
- Ferrari R., Ceconi C., Curello S., et al. Occurrence of oxidative stress during myocardial reperfusion. Mol Cell Biolchem 1992; 111(1–2): 61–69.
- Garden D.L., Granger D.N. Pathophysiology of ishaemia-reperfusion injury. J Pathology 2000; 190(3): 255–266, http://dx.doi.org/10.1002/(SICI)1096-9896(200002)190:3255::AID-PATH5263.0.CO;2-6.
- Rolfe D.F.S., Brown G.C. Cellular energy utilization and molecular origin of standard metabolic rate in mammals. Physiol Rev 1997; 77(3): 732–758.
- Yakovlev A.Yu., Kichin V.V., Nikol’skiy V.O., et al. The effectiveness of isotonic Sterofundin after acute massive blood loss in experiment. Obshchaya reanimatologiya 2013; 9(3): 24–29.
- Yakovlev A.Yu., Emel’yanov N.V., Mukhina I.V., et al. The choice of infusion solution to prevent multiorgan failure in acute massive blood loss (an experimental study). Obshchaya reanimatologiya 2010; 6(3): 48–51.
- Yakovlev A.Yu., Kalent'ev G.V., Snopova L.B., et al. Infusion prevention of myocardium injuries in case of acute massive blood loss (experimental research). Meditsinskiy al’manakh 2012; 5: 191–193.
- Vashetko R.V., Pronin O.V. K patologicheskoy anatomii nekotorykh vnutrennikh organov pri travmaticheskom shoke. V kn.: Sbornik trudov NII SP im. I.I. Dzhanelidze [About pathological anatomy of some internal organs in traumatic shock. In: Collected works of I.I. Dzhanelidze Research Institute of Emergency]. Leningrad; 1978; р. 56–60.
- Vorob’ev A.V., Razumovskiy A.V., Bukhvalov S.A., et al. Dynamics of adult traumatism and the state of specialized medical care in traumas in Nizhny Novgorod region (1995–2008). Meditsinskiy al’manakh 2009; 4(9): 20–25.
- Agadzhanyan V.V., Ust'yantseva I.M. Scientific and practical concept of treatment of polytraumas.Politravma 2013; 2: 5–10.
- Lobo D.N., Dube M.G., Neal K.R., et al. Problems with solutions: drowning in the brine of an inadequate knowledge base. Clin Nutr 2001; 20(2): 125–130, http://dx.doi.org//10.1054/clnu.2000.0154.
- Zander R., Adams H.A., Boldt J., et al. Requirements and expectations for optimal volume replacement. Anasthesiol Intensivmed Notfall Schmerzther 2005; 40(12): 701–719.
- Kentner R., Safar P., Behringer W., et al. Early antioxidant therapy with tempol during hemorrhagic shock increases survival in rats. J Trauma 2002; 53(5): 968–977.
- Gorbacheva I.A., Sycheva Yu.A., Slepneva L.V., et al. Antihypoxic therapy capabilities in the management of patients with coronary heart disease. Profilakticheskaya i klinicheskaya meditsina: vestnik Sankt-Peterburgskoy gosudarstvennoy meditsinskoy akademii im. I.I. Mechnikova 2010; 2(35): 166–176.
- Sofronov G.A., Selivanov E.A., Khanevich M.D., et al. The use of antihypoxic infusion solutions in surgery. Vestnik Natsional’nogo mediko-khirurgicheskogo tsentra im. N.I. Pirogova 2011; 1(6): 87–91.