Biological and Prognostic Role of Tumor Size (T1a, T1b, T1c) in Stage I Breast Cancer (Experience of International Cooperation)
The aim of the investigation was to study shared distribution of tumors T1a, T1b and T1c in two independent female populations: in the country with National breast cancer screening program (the Netherlands) and in the country with voluntary mammographic screening program (Russia), and assess biological and prognostic value of tumor size for the risk of further disease progression and cancer-related mortality.
Materials and Methods. We studied stage I breast cancer (T1N0M0) heterogeneity in Russian and Dutch female population. The study involved Russian women who had undergone radical treatment in N.N. Blokhin Russian Cancer Research Center of Russian Academy of Medical Science and Clinic of Russian Medical Academy for Postgraduate Education (n=1341), Dutch women who had received treatment in LUMC (n=553) and the patients included in National Cancer Register of the Netherlands (n=22196).
Results. Percentage of tumors <10 mm (T1a and T1b) in Dutch women is significantly higher (to 34%) compared with that in Russian women (17.1%) that can be explained by the differences in breast cancer screening in these countries. Stage I breast cancer is a heterogeneous group with a favorable course, if microcarcinomas are <5 mm, and more aggressive — in tumors T1b and T1c. Only microcarcinomas T1a have a favorable biological “portrait” (high percentage of luminal А-subtype tumors with low anaplasia degree) that has an impact on long-term treatment results (minimum recurrence rate and mortality rate and best long-term survival rate). Biological characteristics of tumors T1b and T1c are more aggressive and have higher percentage of ductal breast cancer with luminal В-subtype and triple negative cancer immunophenotype that significantly worsens the disease prognosis.
According to GLOBOCAN (a co-project of World Health Organization and International Agency for Research on Cancer in 180 countries in the world) in 2012 over 1.67 million patients with breast cancer (BC) were diagnosed worldwide [1]. BC ranks first (25% of all malignancies) in morbidity pattern in female population; as well as it takes the lead in mortality (14.3%) among women worldwide, and ranks next to lung cancer in developed countries. The incidence varies greatly: from 25 per 100 000 women in Africa to 96 per 100 000 — in Western Europe. BC incidence is steadily increasing both in developed and developing countries; active early cancer detection programs — screening — takes on enormous importance in an increased number of newly diagnosed cases [2]. Screening — breast examination in potentially healthy female population aimed at early breast cancer detection — was first introduced in the USA, and then in some developed countries (UK, Canada, Netherlands) [3–6]. With the advent of screening programs, there appeared a term “screening” cancer, a tumor of minimum size (as a rule, less than 1.0 cm), with no clinical manifestations and detected only by special instrumental diagnostic techniques. Regular screening mamographies enabled to diagnose 85–90% of all tumors at “screening” stage that made it possible to redistribute BC stages in general female population towards the growth of stage I cancer cases, and ultimately, significantly reduce the mortality rate from this malignant pathology [7–10]. In highly developed countries with introduced national compulsory screening programs for female population (Netherlands, Denmark, UK, USA, Canada) BC mortality “peak” was recorded in 1985–1995 followed by significant decrease of mortality rate. By contrast, in countries with no compulsory programs, but having voluntary screening (including Russia), BC mortality rate is increasing despite highly effective verifying diagnostic programs and extensive use of adjuvant medical therapy [1]. Some researchers [11–14] study a prognostic role of primary tumor size at early BC stages, however, they distinguish the subgroups by the disease stages (I–II) neglecting the heterogeneity of stage I including both microcarcinomas (tumors less than 5 mm — T1a), and tumors of 5–10 mm (T1b) and 10–20 mm (T1c). Biological characteristics and prognostic role of a tumor size (T1a, T1b and T1c) in patients with stage I BC are still unstudied, however, these factors are of primary importance for adjuvant systemic therapy [12]; and that is the background for our survey aimed at studying stage I BC heterogeneity structure.
The aim of the investigation was to study shared distribution of tumors T1a, T1b and T1c in two independent female populations: in the country with National breast cancer screening program (the Netherlands) and in the country with voluntary mammographic screening program (Russia), and assess biological and prognostic value of tumor size for the risk of further disease progression and cancer-related mortality.
Materials and Methods. The international study involved the patients with stage I BC (T1N0M0) after radical treatment (radical mastectomy or breast conserving therapy with or without adjuvant systemic and/or radiotherapy) in Russia (N.N. Blokhin Russian Cancer Research Center of Russian Academy of Medical Science, Clinic of Russian Medical Academy for Postgraduate Education — 1341 female patients) and the Netherlands (LUMC — 553 patients) provided from 1985 to 2009. For comparison we used National Cancer Register data of the Netherlands including all women with stage I BC who underwent radical treatment in Holland from 1989 to 2009 (22196 women). The age of Russian patients involved in the survey was 26–88 years (median — 53 years); under 40-year-old women amounting to 12.1%; at the age of 40–50 years — 26.7%; 50–60 years — 29.2%; over 60 years — 32.1%. Dutch patients treated in LUMC were older (p<0,001): age median — 56 years (23–89 years), percentage of patients under 40 years was 8.3%, 40–50 years — 19.5%, over 60 years — 39.6%. Dutch women included in National Cancer Register appeared to be significantly older: age median — 62 years (19–101 years), and percentage of women under 40 years was as little as 5.1%, women aged 40–50 years — 16%, and more than a half of patients (55.6%) were over 60 years.
According to elective morphological studies, infiltrative ductal carcinoma prevails (in Russian women — 81.2%; in LUMC patients — 89.9%; in patients inclu ded in Cancer Register — 88.6%, p>0.05), however, anaplasia degree of tumors among Russian and Dutch women was different. So, high-differentiated tumors (G1) were detected in 13.8% Russian patients, in LUMC patients — 20.5%; among women included in Cancer Register — 24.3% cases; and G2–3 tumors were found in most Russian women (86.2%), in 79.5% LUMC patients, and in 75.7% Dutch women, p<0.001. Receptor status assessment showed the predominance of hormone-positive tumors in both female populations: in Russian patients estrogen-positive (ER+) tumors were found in 74.1% cases, in LUMC patients — 78.4%, and significantly more frequently in general Dutch female population — 86.4%; p<0.001. Progesterone receptor status assessment revealed identical predominance of PR+ tumors in Russian (71.6%) and Dutch (70.6%) women, p>0.05; though the percentage of PR+ tumors in LUMC tumors was significantly lower (55.4%), p<0.001.
We had the data on HER2-status and proliferation index Ki-67 of Russian patients only. HER2 hyperexpression (HER2+++, HER2++ and amplification at FISH-reaction) was revealed in 9.7% cases. No HER2 hyperexpression (HER0, HER1+ in immunohistochemistry (IHC), as well as HER2++ in the absence of amplification at FISH-reaction) was found in 90.3%. IHC showed HER2++ hyperexpression in 12 patients, but FISH-reaction was not carried out due to various reasons; so it is impossible to interpret HER2-status as positive or negative. Proliferation index Ki-67 in Russian patients was rendered as “high” if Ki-67 >20% (according to St. Gallen, 2013, and RUSSCO, 2013, recommendations) in 57.9% patients, and as “low” t— if Ki-67 <20% — in 42.1% cases.
Based on receptor status (ER, PR, HER2 and Ki-67) in Russian patients we distinguished 5 immunophenotypic tumor subtypes. Luminal А-subtype (ER+PR+HER2–, Ki-67 <20% or G1) prevailed in 36% cases; luminal В HER2-negative subtype (ER+PR+HER2–, Ki67 >20% or G3) was found in 25.2% women; luminal В HER2-positive subtype (ER+PR+HER2+, any Ki-67 or G) was revealed in 9.6% cases; triple negative breast cancer (ER–PR–HER2–, any Ki-67 or G) — in 22.9%; non-luminal HER2+ subtype (ER–PR–HER2+, any Ki-67 or G) — in 6.3% cases (Table 1).
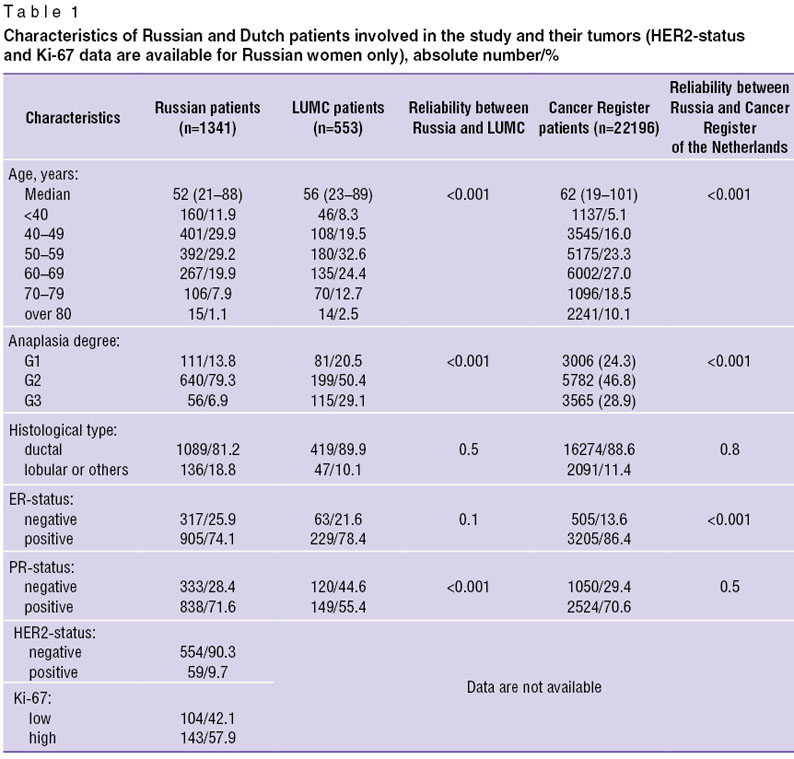 Table 1. Characteristics of Russian and Dutch patients involved in the study and their tumors (HER2-status and Ki-67 data are available for Russian women only), absolute number/% Table 1. Characteristics of Russian and Dutch patients involved in the study and their tumors (HER2-status and Ki-67 data are available for Russian women only), absolute number/%
|
According to primary tumor size the patients were divided into three groups: group 1 — women with tumors up to 5 mm in size (T1a); group 2 — with 5–10 mm tumors (T1b); group 3 — with 10–20 mm tumors (T1c). We compared tumor size distribution in Russian and Dutch women. In Russian female population we studied biological characteristics of different tumor sizes (T1a, T1b, T1c), prognostic value of tumor size for further progression and death, as well as the distribution of tumor sizes among women who had undergone treatment in different periods of time.
The findings were statistically processed using international statistical program SPSS 20.0; for comparative analysis in the study groups we applied univariable analysis using Pearson χ2; survival indices were determined by Kaplan–Meier method, and then controlled by Cox regression analysis. The differences were reliable if p<0.05.
This retrospective study was approved by the Ethic Committee of N.N. Blokhin Russian Cancer Research Center of Russian Academy of Medical Science and it complies with the declaration of Helsinki (adopted in June, 1964 (Helsinki, Finland) and revised in October, 2000 (Edinburg, Scotland)). All patients admitted to Russian Cancer Research Center, Clinic of Russian Medical Academy for Postgraduate Education and LUMC gave their consent to apply their data for scientific purposes.
Results
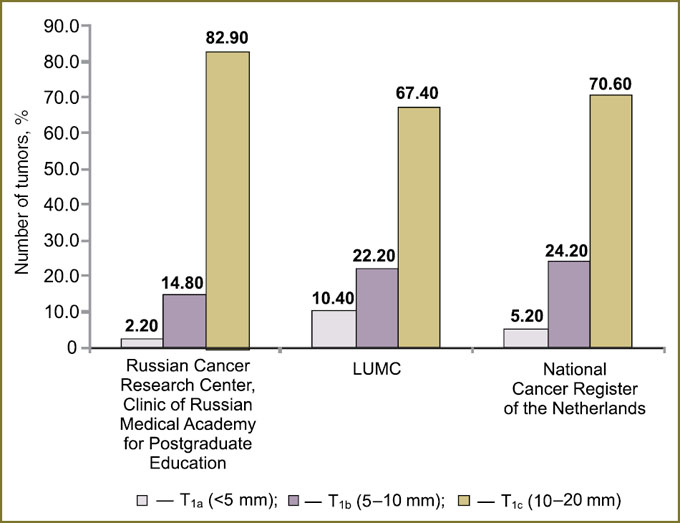
Tumor size in Russian and Dutch female populations. The comparison of primary tumor size in Russian and Dutch women showed statistically significant differences (p<0.001). The third of Dutch women had tumors less than 10 mm (T1a and T1b), while the percentage among Russian women was only 17.1%. Microcarcinomas <5 mm (T1a) were found in Russian patients in 2.2% cases, and diagnosed in Dutch patients significantly more frequently (LUMC — 10.4%; Cancer Register data — 5.2%). The percentage of 5–10 mm tumors was minimal (14.8%) in Russian population and significantly higher among Dutch women (LUMC — 22.2%; Cancer Register data — 24.2%). 10–20 mm tumors (T1c) prevailed among Russian patients (82.9%), and among LUMC patients they were 67.4%, and 70.6% — in all Dutch women (Fig. 1).
|
|
Thus, there were found statistically significant differences in microcarcinoma distribution among Russian and Dutch women (p<0.001). High percentage (up to 34%) of tumors <10 mm (T1a and T1b) among Dutch patients with stage I BC is the result of the well organized fulfillment of the national screening program in the Netherlands, while the lack of such a program in Russia can explain low percentage of such tumors in Russia (only 17.1%).
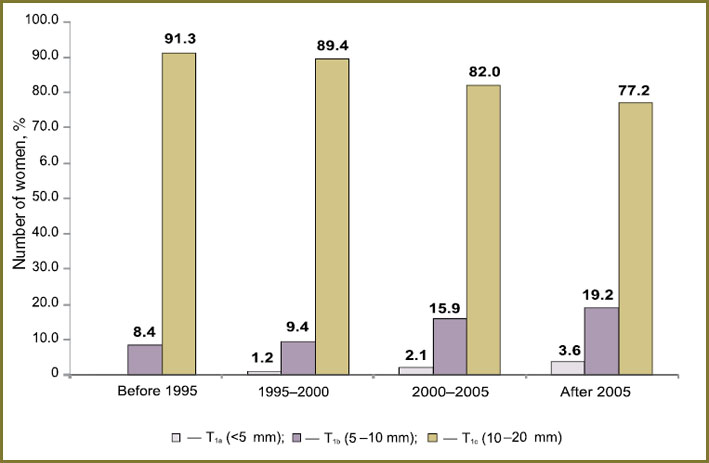
Tumor size dynamics from 1985 to 2012 in Russian female population. We revealed tumor size (T1a, T1b and T1c) distribution among Russian women who had received treatment in different periods of time: from 1985 to 1995 — 309 patients, 23.0%; from 1995 to 2000 — 160 patients, 11.9%; from 2000 to 2005 — 289 patients, 21.6%; from 2005 to 2012 — 583 patients, 43.5%. There was found progressive growth of “small” tumors (T1a and T1b) as time passed: the percentage of tumors <5 mm (T1a) among the women who received treatment before 1995 was low (0.3%), and such tumors were diagnosed in 3.6% of those women who underwent treatment after 2005; and the percentage of 5–10 mm tumors (T1b) increased from 8.4% (before 1995) to 19.2% (after 2005). Therefore, the number of 10–20 mm tumors (T1c) reduced from 91.3% to 77.2% (p<0.0001) (Fig. 2).
|
|
Thus, as time passes there is a significant increase of microcarcinomas (tumors T1a and T1b) and reduction of T1c tumors, that is likely to result from improved instrumental diagnostics (mammography and ultrasound).
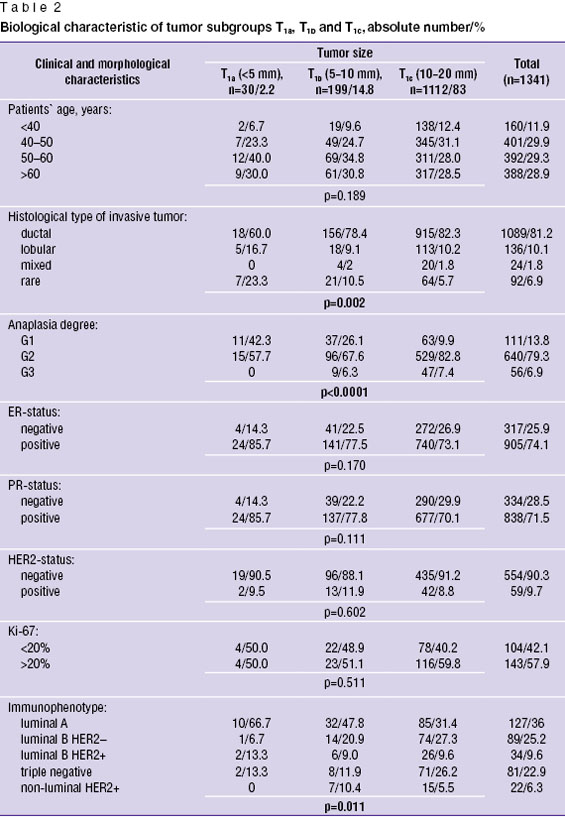
Biological significance of tumor size in Russian female population. The comparison of age and morphological characteristics in different primary tumor size (T1a, T1b and T1c) in 1341 Russian patients showed significant biological differences in tumor subgroups. So, patients′ age, ER, PR, HER2 status and Ki-67 had no significant differences in different tumor sizes (p>0.05), however, histological cancer type, tumor grade and tumor phenotype clearly correlated to carcinoma size (p<0.05). The percentage of ductal cancer progressively grew with the increase of primary tumor size (in T1a tumors — 60%, in T1b — 78.4%, and in T1c — 82.3%), while the percentage of rare favorable tumors (tubular, papillary carcinomas) was maximum (23.3%) in tumors <5 mm, and amounted only to 5.7% in tumors T1c (p=0.002). Anaplasia degree also essentially depended on carcinoma size: the percentage of high-differentiated tumors G1 in tumors <5 mm was maximum (42.3%) and minimum — in 10–20 mm carcinomas (9.9%). In addition, there were no low-grade tumors G3 in patients with tumors T1a, while patients with T1b (6.3%) and T1c (7.4%) tumors, p<0.0001, had such carcinomas (Table 2).
|
|
Tumor phenotype comparison revealed significant predominance of luminal А-subtype among women with tumors <5 mm (66.7%), and with the increase of tumor size the percentage of A-subtype significantly decreased (in tumors T1b — 47.8%; T1c — 31.4%). In contrast, the percentage of HER2-negative luminal B-subtype was minimum in microcarcinomas <5 mm (6.7%), increased up to 20.9% if tumor size was 5–10 mm, and reached its maximum in tumors 10–20 mm — 27.3%. Similar significant differences were found in patients with triple negative cancer: in tumor T1a — 13.3%, in T1b — 11.9%, and in T1c — 26.2% (p=0.011). It is notable that HER2+ non-luminal subtype was detected only in women with T1b and T1c tumors, while it was missing in those with microcarcinoma T1a.
Thus, there are essential biological differences in tumor subtypes T1a, T1b and T1c. The most favorable characteristics (low-grade luminal А-subtype prevailing) were found in tumors <5 mm, and the percentage of unfavorable tumor subtypes (luminal B-subtype and triple negative) — in larger tumors (10–20 mm). Such biological characteristics can also cause different prognostic values of tumor size in stage I BC.
Prognostic value of primary tumor size (T1a, T1b and T1c) in Russian female population. Survival analysis (recurrence-free survival — RFS; overall survival — OS; cancer-specific survival — CSS) involved Russian patients with at least a 36-month-follow up (RFS — 1167 patients; OS — 1209; CSS — 1182 women). In median equal to 75 months (6–312 months) recurrences were revealed in 255 patients (21.9%); among them local recurrences were 32.5% (83 cases), regional recurrences — 6.7% (17 cases) and distant metastases were detected in 60.8% (155 patients). Time to disease progression was 6–204 months, median — 36 months. During a follow up period 197 women (16.3%) died; most of them (169 women, 85.8%) — of progressive cancer, and in 28 cases (14.2%) — of other reasons.
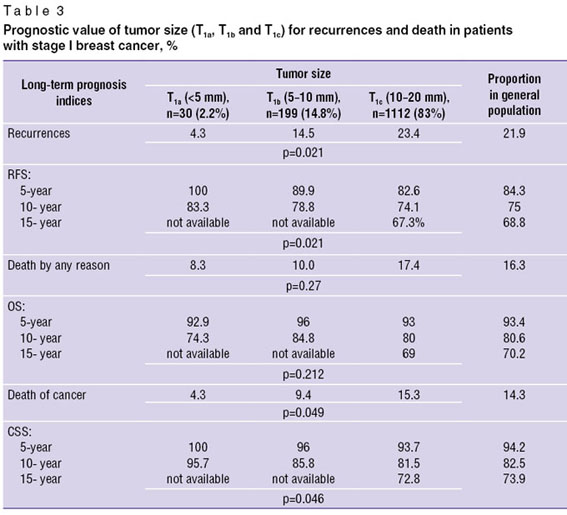
The percentage of recurrences in subgroups of women with T1a, T1b and T1с tumors differed significantly (p=0.021): in patients with T1а microcarcinomas further progression was observed only in 4.3% cases, in T1b tumors — in 14.5% cases, and significantly higher (23.4%) — in patients with T1c tumors. The differences between the groups under study the mortality rate of other causes did not reach statistical significance (p=0.2), however, the analysis of cancer-related deaths revealed essential prognostic regularity (p=0.049): the number of female patients died from further BC progression grows with the increase of tumor size. Moreover, the percentage of women with microcarcinoma T1a is minimum (4.3%), with T1b tumors — 9.4%, and with T1c tumors — 15.3% (Table 3).
|
|
The differences in recurrence and cancer-related mortality rates between the subgroups of women with different tumor sizes were proved by further survival analysis of the patients. So, best RFS indices were observed in patients with microcarcinomas <5 mm (5-year — 100%, 10-year — 83.3%), in patients with tumors 5–10 mm 5-year RFS was 89.9%, 10-year — 78.8% and it was significantly lower if tumors were 10–20 mm (5-year — 82.6%, 10-year — 74.1%), p=0.021. The comparison of OS indices showed no significant differences between the subgroups: 5- and 10-year OS in women with tumors T1a was 92.9 and 74.3%; in patients with T1b carcinomas — 96% and 84.8%, and in women with T1c tumors — 93% and 80%, respectively (p=0.212). However, the comparison of CSS indices evidenced prognostic value of tumor size for risk of death from cancer progression: 5- and 10-year CSS was maximum only in microcarcinomas T1a (100% and 95.7%) and was significantly lower in T1b (96 and 85.8%) and T1c (93.7% and 81.5%) tumors, p=0.046. It is important that statistically significant differences in survival rate (RFS and CSS) in different tumor sizes arise as early as by a 5-year follow up and become more significant 10 years later. There are 15-year survival indices only for women with T1c tumors: 15-year RFS — 67.3%, OS — 69% and CSS — 72.8%; the percentage of patients with T1a and T1b tumors who underwent treatment before 2000 is minimum and insignificant for statistical analysis of 15-year survival.
Conclusion. Stage I breast cancer is a heterogeneous group with a favorable course in microcarcinomas <5 mm, and more aggressive — in tumors T1b and T1c. Only microcarcinomas T1a have a favorable biological “portrait” (high percentage of luminal А-subtype tumors with low anaplasia degree) that has an impact on long-term treatment results (minimum recurrence rate and mortality rate and best recurrence-free and cancer-specific survival). Biological characteristics of tumors T1b and T1c are more aggressive and have higher percentage of ductal breast cancer with luminal В-subtype and triple negative cancer immunophenotype that significantly worsens the disease prognosis. Microcarcinomas (T1a and T1b) detected at pre-existing disease stage amount to 34% among Dutch women due to National screening program; while among Russian women the percentage of such tumors is only 17.1% that reflects the failure of the existing breast cancer diagnostic programs in Russia. The obtained results support the world literature data on a positive role of mammographic screening in breast cancer [1, 2, 7].
Study Funding and Conflict of Interests. The study was not supported by any funds, and the authors have no conflict of interest to disclose.
References
- GLOBOСAN 2012. http://globocan.iarc.fr.
- Saika K., Sobue T. Cancer statistics in the world. Gan To Kagaku Ryoho 2013 Dec; 40(13): 2475–2480.
- Thomas B.A., Price J.L., Boulter P.S., Gibbs N.M. The first three years of the Guildford Breast Screening Project. Recent Results Cancer Res 1984; 90: 195–199.
- Goin J.E., Haberman J.D., Linder M.K., Lambird P.A. Analysis of mammography: a blind interpretation of BCDDP radiographs. Radiology 1983 Aug; 148(2): 393–396.
- Brisson J., Morrison A.S., Khalid N. Mammographic parenchymal features and breast cancer in the breast cancer detection demonstration project. J Natl Cancer Inst 1988 Dec 7; 80(19): 1534–1540, http://dx.doi.org/10.1093/jnci/80.19.1534.
- Smart C.R., Byrne C., Smith R.A., et al. Twenty-year follow-up of the breast cancers diagnosed during the Breast Cancer Detection Demonstration Project. CA Cancer J Clin 1997 May–Jun; 47(3): 134–149.
- Tabar L., Yen M.F., Vitak B., et al. Mammography service screening and mortality in breast cancer patients: 20-year follow-up before and after introduction of screening. Lancet 2003; 361: 1405–1410, http://dx.doi.org/10.1016/S0140-6736(03)13143-1.
- Gotzsche P.C., Olsen O. Is screening for breast cancer with mammography justifiable? Lancet 2000; 355: 129–134, http://dx.doi.org/10.1016/S0140-6736(99)06065-1.
- Health Quality Ontario. Screening mammography for women aged 40 to 49 years at average risk for breast cancer: an evidence-based analysis. Ont Health Technol Assess Ser 2007; 7(1): 1–32.
- Weisstock C.R., Rajapakshe R., Bitgood C., et al. Assessing the breast cancer risk distribution for women undergoing screening in British Columbia. Cancer Prev Res 2013 Oct; 6(10): 1084–1092, http://dx.doi.org/10.1158/1940-6207.CAPR-13-0027.
- Mohammed Z.M., McMillan D.C., Edwards J., Mallon E., Doughty J.C., Orange C., Going J.J. The relationship between lymphovascular invasion and angiogenesis, hormone receptors, cell proliferation and survival in patients with primary operable invasive ductal breast cancer. BMC Clin Pathol 2013 Nov 25; 13(1): 31, http://dx.doi.org/10.1186/1472-6890-13-31.
- Uchida N., Suda T., Ishiguro K. Effect of chemotherapy for luminal a breast cancer. Yonago Acta Med 2013 Jun; 56(2): 51–56.
- Rouanet P., Roger P., Rousseau E., Thibault S., Romieu G., Mathieu A., Cretin J., Barneon G., Granier M., Maran-Gonzalez A., Daures J.P., Boissiere F., Bibeau F. HER2 overexpression a major risk factor for recurrence in pT1a-bN0M0 breast cancer: results from a French regional cohort. Cancer Med 2014 Jan 10, http://dx.doi.org/10.1002/cam4.167.
- Comen E.A., Norton L., Massague J. Breast cancer tumor size, nodal status, and prognosis: biology trumps anatomy. J Clinical Oncology 2011; 2619–2622, http://dx.doi.org/10.1200/JCO.2011.36.1873.