Characteristics of Left Ventricular Impaired Functional Indices in Patients with Coronary Heart Disease According to Visual Estimation and Velocity Vector Imaging
The aim of the investigation was to estimate the diagnostic capabilities of left ventricular (LV) functional indices in patients with coronary heart disease (CHD) using conventional imaging techniques (echoCG) and VVI technology.
Materials and Methods. 52 patients with CHD were examined. By visual estimation (echoCG) of LV segmental contractility all patients were divided into two groups: without LV contractile dysfunction (n=26); with segmental contractile dysfunction (n=26).
The investigation of LV function using VVI system included the study of longitudinal, radial and circular LV fibers, and the analysis of rotation indices.
Results and Discussion. VVI system helped to reveal in all patients systolic dysfunction and abnormal strain rate of LV myocardium. Patients of both groups were found to have dysfunction of longitudinal and circular fibers of LV myocardium. Decreased indices of radial fiber function were recorded in a group of patients with segmental contractility dysfunction.
Rotation function analysis was impossible in visual estimation. VVI application enabled to find disturbed rotation of basal and apical LV parts. So, the patients of both groups had decreased apical rotation indices, and 14 of them were recorded to have disturbed apical and basal rotational direction of LV.
Conclusion. The use of VVI system enables to study in more detail the characteristics of LV function in CHD patients and reveal the alteration of those indices which are not found in visual control. The detection of disturbed strain and rotational properties of LV myocardium is the most urgent problem in patients with regular contractility that makes it possible to define well the management of such patients.
One of the main reasons of coronary heart disease (CHD) development is atherosclerotic damage of coronary arteries caused by dysfunction of endothelium, dyslipidemia, and disturbances of rheological blood properties [1–4].
Dysfunction of the left ventricular (LV) myocardium in CHD is caused by the presence of scar changes and the degree of their intensity, as well as ischemic and post-ischemic alterations of its contractility, the degree of coronary artery lesions, diastolic and systolic function [5–7].
Standard echocardiographic (echoCG) examination of the CHD, including the assessment of systolic, diastolic and contractility LV function, determines objectively enough functional capabilities of LV myocardium. The evaluation of systolic LV function is performed in B-mode by means of the modified Simpson method. Assessment of the contractile function is based on the analysis of LV segment movement. According to the recommendations of the American Association of Echocardiography (1989), LV is divided into 16 segments: 6 basal and middle, and 4 apical [8]. In 17-segment model, developed in 2002, the 17th segment of LV is the heart apex itself [9]. Both of these segment models are used in the clinical practice. According to the recommendations, the 17-segment model should be used in studying myocardial perfusion and in comparing different visualization techniques [9].
In a number of patients with CHD and/or after myocardial infarction, zones of local contractility impairment at rest may not be revealed during visual assessment (echoCG). However there are patients in which during visual control the volume of ischemized myocardium may be increased due to the disturbances in adjacent segment kinetics caused by pull-up phenomenon and changes of load conditions, and myocardium stunning [8, 9].
A more objective assessment of LV function in patients with IHD may be reached using technologies of tissue Doppler examination, Speckle Tracking and Velocity Vector Imaging (VVI). Despite of the fact, that tissue Doppler echoCG possesses high sensitivity in revealing low-speed segment movement, facilitates early diagnosis of minimal functional LV changes, that cannot be revealed with the help of indexes used in echocardiography, and also enables the cardiologists to study successfully regional LV function, the accuracy of this method is limited by the scanning angle and availability of artifacts and reverberations [10, 11].
In contrast to the tissue dopplerography, Speckle Tracking method is based on determining the velocity of myocardial movement by tracking the dislocation of the so-called spotted structures on the standard echocardiographic image in B-mode which allows to obtain information on the velocity, deformation (strain) and velocity of deformation of the areas surrounding myocardium without restrictions connected with the parallelism of the object and US beam movement. This method does not have some of the limitations and drawbacks of Doppler tissue visualization, is less labor-consuming and more applicable for clinical practice [10, 12]. The data obtained may be presented in the form of diagrams resembling a bovine eye. Deformation myocardial properties and LV rotation can be clearly estimated by them [10, 12, 13].
A great number of works is devoted to the clinical application of Speckle Tracking in patients with CHD in the Russian and foreign literature [12, 14–23].
The technology of visualizing myocardium movement velocity vector (Velocity Vector Imaging) uses not only the principle of tracking speckle structures (just like Speckle Tracking) but also the technology of matching the global movement with the periodicity of periodicity of cardiac cycles [24]. This methodology makes it possible to perform a more complicated analysis, comprising endocardium tracking, performed on the basis of Fourier analysis, providing higher precision of heart movement measurement for reliable and quantitative evaluation of global and regional myocardium function [25]. This provides information on direction and value of myocardial movement velocity vector during the whole cardiac cycle. This method allows the assessment of velocity, strain, strain rate, systolic and diastolic function of the heart ventricles along the short and long axis without angular limitations, and also analysis of myocardium dyssynchrony and rotation data. The possibility of evaluating functional indices of LV or any other cardiac cavity at any point is the advantage of this method.
There are much less publications devoted to the clinical significance of VVI technique in the literature [10, 24–26].
The aim of the investigation is to evaluate the feasibilities of diagnosing functional parameters of the left ventricle in patients with coronary heart disease using traditional visualization technique (echoCG) and VVI method.
Materials and Methods. 52 patients with СHD (4 females and 48 males) treated in the Specialized Cardiologic Clinical Hospital of Nizhny Novgorod (Russia) were examined. The average age was 52±6 years (from 38 to 57 years). Myocardial infarction was in the history of 38 patients, in 14 patients only ischemic changes were registered.
The study complies with the Declaration of Helsinki (the Declaration was passed in Helsinki, Finland, June, 1964, and revised in October, 2000, Edinburg, Scotland) and was performed following approval by the ethic committee of Nizhny Novgorod State Medical Academy. Written informed consent was obtained from every patient.
Echocardiographic examination was performed using ultrasound scanner Acuson X 300 (Siemens, Germany) with 1–5 MHz transducer in B-mode, in the mode of Doppler blood flow study and color Doppler mapping. The analysis of deformation LV myocardium properties was carried on in the post-procession mode by means of Syngo VVI (Siemens Medical Solutions USA Inc., USA).
The analysis of LV systolic function during standard echographic examination was made according to the modified Simpson method. Volumes of LV — (end diastolic (EDV) and end systolic volume (ESV)), LV ejection fraction (EF), stroke volume (SV) in the apical 4-chamber and apical 2-chamber positions were calculated. For more objective LV remodeling analysis index of sphericity and indices of LV volumes were quantified. The assessment of segmental contractility at rest was performed according to the recommendations of the American Association of Echocardiography dividing LV into 16 segments. Local contractility impairment index (LCII) was calculated.
Studying LV function by means of VVI system, longitudinal, radial and circular LV fibers were evaluated. The analysis of longitudinal, radial and circular strain and strain rate was carried on. In the transversal LV sections data of rotation were analyzed at the level of basal and apical areas.
On the basis of visual assessment (echoCG) of segmental LV contractility all patients were separated into 2 groups: group 1 (n=26) included patients without impairment of contractility LV function; group 2 (n=26) consisted of patients with segmental contractility impairment. LCII in group 1 was equal to 1. LCII in group 2 was, in the average, 1.64±0.45 (from 1.12 to 2.5).
Data were statistically processed by the program Statistica 6.0 using Student criteria.
Results and Discussion. Analysis of LV systolic function revealed statistically significant difference between the two groups when comparing LV volumes, LV volume indices and EF in 4- and 2-chamber positions. Comparison of systolic and diastolic sphericity indexes (SI) and SV value in the groups did not show any statistically significant difference (Table 1).
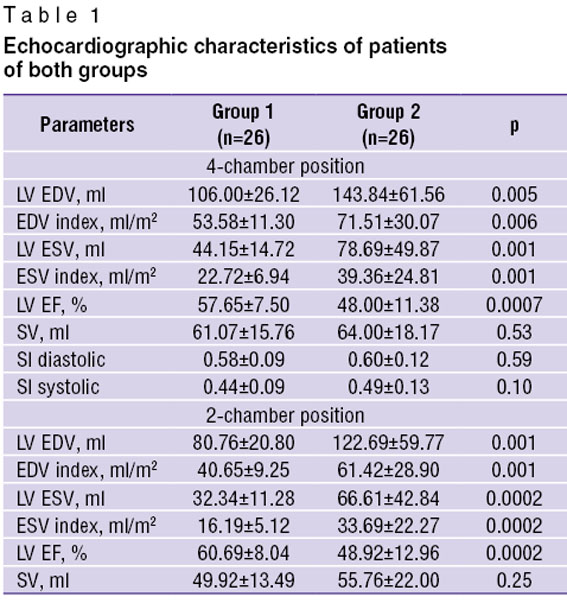 Table 1. Echocardiographic characteristics of patients of both groups Table 1. Echocardiographic characteristics of patients of both groups
|
Patients suffered Q-myocardial infarction (23 persons) dominated in group 2. In 9 of them post-infarction LV aneurism had been formed. 2 patients of this group had other than Q-infarction in the history, in 1 only ischemic changes were registered by ECG. In group 1 ECG data showed only ischemic changes in 13 patients, and among the remaining 13 people ECG revealed Q-infarction in 7, non-Q-infarction — in 6 examined patients.
All patients underwent transcutaneous coronary intervention (TCI). The analysis of the damaged coronary bloodstream areas (Table 2) found no essential difference between them in the groups. The number of patients with the damage of the left coronary artery (LCA) trunk in group 2 was a bit more (5 people) than in group 1 (3 patients).
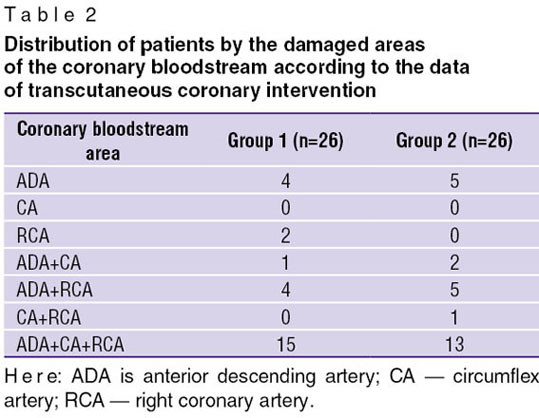 Table 2. Distribution of patients by the damaged areas of the coronary bloodstream according to the data of transcutaneous coronary intervention Table 2. Distribution of patients by the damaged areas of the coronary bloodstream according to the data of transcutaneous coronary intervention
|
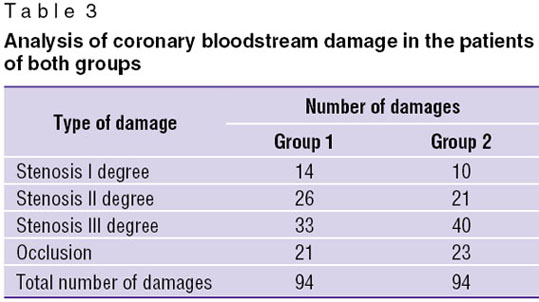
The analysis of the damaged coronary bloodstream also did not reveal significant difference between the two groups (Table 3).
|
|
A somewhat more cases of stenosis III degree were found out in group 2, while a number of cases with occlusions, stenoses I and II degree does not differ much. Thus, reliable differences in the volume and character of coronary vessel damage were not revealed in patients of both groups.
Strain (S) of the myocardium fibers is a change of their length (shift) during contraction and relaxation of myocardium, expressed in percent. In other words, strain is an object deformation relative to its initial length [27]. Shortening is presented by negative values, lengthening — by positive ones [13]. Consequently, shortening of the longitudinal and circular fibers in the systole phase is expressed by negative values, and lengthening of the radial fibers — by positive ones. Longitudinal contraction reflects actually the pumping work of LV in the longitudinal axis. Normally, systolic strain of myocardial fibers equals in the average, to 20%. Decrease of this value is a marker of the deformation impairment. Thus, a systolic strain or deformation shows the change of the thickness or length of the wall segment.
Velocity, with which this change occurs, or the so-called deformation gradient, is measured by a strain rate value (SR, s–1) or velocity of deformation [27, 28]. According to the authors [13, 29, 30] strain rate is the velocity of mutual displacement of two points located at a fixed distance. Notably, that graphic image of strain rate has a systolic and diastolic component. Thus, normally, shortening of the myocardium longitudinal fibers (negative strain rate) takes place during systole, in diastole their elongation occurs (positive strain rate) [31, 32]. Radial and circular strain rates are studied in short parasternal sections. Radial strain rate is the rate of thickening myocardium segments in systole (positive strain rate), and decrease of thickness in diastole (negative strain rate). Circular strain rate reflects shortening of myocardium fibers in systole (negative strain rate), and their elongation in diastole (positive strain rate) [27].
In the given investigation according to VVI data decrease of indices of longitudinal and circular systolic strain was observed in all patients (Table 4).
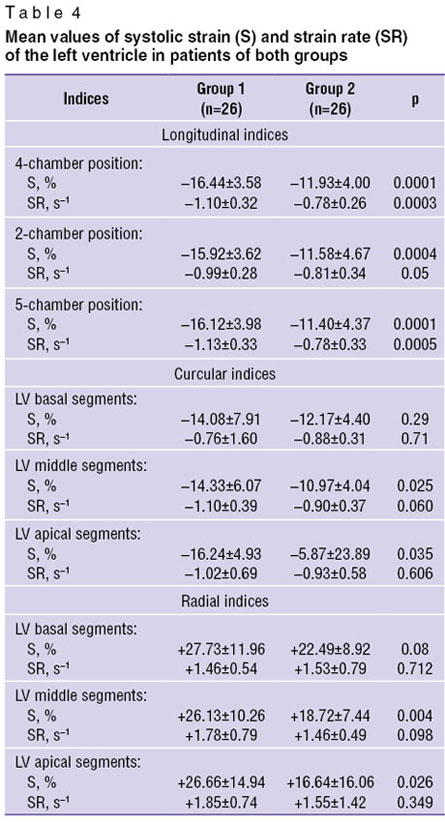 Table 4. Mean values of systolic strain (S) and strain rate (SR) of the left ventricle in patients of both groups Table 4. Mean values of systolic strain (S) and strain rate (SR) of the left ventricle in patients of both groups
|
Values of longitudinal systolic strain were statistically significantly lower in the group of patients with visually revealed impairment of contractility. Statistically significant decrease of circular strain values was found out in the patients of group 2 in the middle and apical segments of LV. Similar to strain values reduction of indices of longitudinal and circular systolic strain rate was established in both groups. Statistically significant decrease of longitudinal SR in group 2 was obtained in the apical 4- and 2-chamber positions. Differences in the values of longitudinal SR in the apical 5-chamber position and circular SR in the transverse positions of LV were not revealed.
Indices of radial strain were within the normal values in group 1. Their statistically significant decrease was noted in group 2 in the middle and apical segments. Statistically significant differences of SR index in both groups were not established. Strain rate index of LV radial fibers was found to be within the norm in all patients. Such difference between SR and S indices of radial fibers is likely to be connected with the specificity of the given indices. Strain reflects no more than alterations of wall thickness (or segment), whereas strain rate shows the rate of this change, i.e. is the velocity gradient of myocardium segment deformation [27]. Two objects, in the judgement of J. D′hooge and A. Heimdal et al. [33, 34], may have similar strain but its different velocity.
Thus, with an estimated difference of the values of visual assessment of LV contractile function in two groups, application of VVI technique showed the reduction of deformation properties of LV myocardium in both groups: with uniform contractility (group 1) and in the group (group 2) with impaired segmental contractility. Besides, in both groups dysfunction of longitudinal and circular LV myocardium fibers was determined. Decrease of radial fiber function was noted in the group of patients with visually impaired contractility (group 2). Diminishing of strain values of all LV myocardium fibers depends, in the opinion of the authors, on the severity of the myocardial infarction suffered. Indeed, the majority of patients from group 2 (23 patients) had Q-infarct in the history. Our suppositions find their confirmation in the literature. In the work [12] it is demonstrated that indices of longitudinal, radial and circular strains decrease in transmural myocardial infarction.
Alongside with the indices of strain and strain rate of separate myocardial segments, VVI method enables the assessment of LV and the whole heart mechanics. Normally LV makes rotation movements, which result in the reduction of longitudinal and radial LV cavity length. The authors [23, 35–37] consider that it is the spiral orientation of LV microfibrills that is the structural basis of LV rotation movement, in which the heart apex goes counterclockwise, and the base — clockwise. The analysis of the graphic rotation characteristics shows that counterclockwise direction of LV rotation has positive values, whereas clockwise direction has negative values, if viewed from the top.
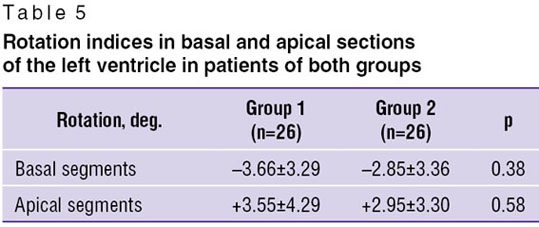
Analyzing the rotation values in the basal and apical sections no statistically significant difference was found in patients of both groups (Table 5).
|
|
In both groups the values of apical rotation were decreased. The detailed analysis showed impairment of rotational mechanics in 14 patients. In 7 of them the apex rotated clockwise (negative values), while in other 7 the base rotated counterclockwise (positive values).
According to the data reported in the literature, rotation indices in the apical segments are higher than in the LV basal ones. Thus, in the study of Sh. Carasso et al. [38] basal endocardial rotation was directed clockwise and a mean value was equal to –3.4±2.0°, whereas index of apical rotation was directed counterclockwise and corresponded to 7.1±3.3°. Absence of difference between the apical and basal rotation indices is also described in the work of E.N. Pavlyukova et al. [23] analyzing patients with CHD and LV EF over 45%.
According to the experimental data of S.T. Toumanidis et al. [22] apical rotation of 2.92° is an early marker of LV systolic dysfunction in acute anterior myocardial infarction with the sensitivity of 80% and specificity 71%, and is also a predictor of EF below 40%.
In the present investigation patients of both groups has a marked damage of the coronary bloodstream and, according to VVI data, decrease of systolic strain and strain rate values. And this, to our opinion, accounts for the reduction of the LV apical rotation indices. Impairment of the rotational mechanics was found out to be in both groups: group 1 — 7 patients, group 2 — 7 patients. These were mainly the patients with 3-vessel damage of the coronal bloodstream. Comparing strain indices, the disturbance of movement direction of radial and circular fibers was found to occur predominantly in the area of the anterior descending artery.
Rotation of the apex counterclockwise (positive values) is mentioned in the works of E.N. Pavlyukova [23], S.D. Solomon et al. [39], and W. Han et al. [40], analyzing patients with acute phase of anterior myocardial infarction with EF below 40%. In our case low indices of radial strain in basal segments were revealed in patients with the apex counterclockwise rotation (n=7), in two of them post-infarction LV aneurism was noted. Among the patients with the counterclockwise rotation of the base (positive values) (n=7) positive values of circular strain in a number of LV segments were defined.
Thus, by visual evaluation of LV contractile function in patients with CHD 2 groups of patients were distinguished: a group with a uniform contractility and with impairment of segmental contractility.
Using VVI system impairments of systolic strain and strain rate of LV myocardium were found in all patients. Dysfunction of longitudinal and circular LV myocardial fibers was determined in both groups. In the group of patients with impaired segmental contractility decrease of radial fiber function values was registered.
It is not possible to analyze rotation function by visual assessment. Application of VVI technique enables revealing disturbances of rotation of basal and apical LV segments. Thus, in patients of both groups apex rotation indices were decreased, and in 14 of them abnormal direction of LV apex and base rotation was recorded.
Conclusion. Application of VVI system makes it possible to study left ventricle function in detail in patients with ischemic heart disease and to detect changes of those indices which cannot be found by visual control. Revealing disorders of rotational and deformation myocardial properties of the left ventricle is especially important in patients with uniform contractility, enabling physicians to define more clearly the tactics of management in this category of patients.
Study Funding and Competing Interest. The study was financed by the authors’ own sources. There is no specific conflict of interest related to the authors of this study.
References
- Oganov R.G., Maslennikova G.Ya. Cardiovascular epidemic can be contained by prevention effort. Profilakticheskaya meditsina 2009; 12(6): 3–7.
- Oganov R.G. Cardiovascular prevention. National recommendations. Kardiovaskulyarnaya terapiya i profilaktika 2011; Suppl. 2; 10(6).
- Quinon J. Relation between extent of dysfunctional yet viable myocardium and improvement in function after revascularization. J Cardiovasc Surg 1998; 48: 124–128.
- Sedov V.M., Mirchuk K.K., Sedlitskiy Yu.I., et al. Dyslipidoproteidemia and prognosis of coronary heart disease after bypass surgery. Vestnik hirurgii 2001; 4: 13–17.
- Bokeriya L.A., Rabotnikov V.S., Buziashvili Yu.I., Chinaliev S.K., Asymbekova E.U., Matskellishvili S.T. Ishemicheskaya bolezn’ serdtsa u bol’nykh s nizkoy sokratitel’noy sposobnost’yu miokarda levogo zheludochka (diagnostika, taktika lecheniya) [Coronary heart disease in patients with low left ventricular myocardial contractility (diagnostics, management)]. Moscow: Izd-vo NTsSSKh im. A.N. Bakuleva RAMN; 2001.
- Liebermann A.N., Weiss J.L., Jugdutt B.I., et al. Relationship of regional wall motion and thickening to the extent of myocardial infarction in the dog. Circulation 1981; 63: 739–746.
- Meza M.F., Kates M.A., Barbee R.W., et al. Combination of dobutamine and myocardial contrast echocardiography to differentiate postischemic from infracted myocardium. J Am Coll Cardiol 1997; 29(5): 274–984, http://dx.doi.org/10.1016/S0735-1097(97)00016-8.
- Lang R.M., Bierig M., Devereux R.B., et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography′s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiography 2005; 18(12): 1440–1463, http://dx.doi.org/10.1016/j.echo.2005.10.005.
- Guidelines for quantitative assessment of heart chamber structure and function. Yu.A. Vasyuk (editor). Rossiyskiy kardiologicheskiy zhurnal 2012; 3(95): 1–28.
- Vasyuk Yu.A. Funktsional’naya diagnostika v kardiologii: klinicheskaya interpretatsiya [Functional diagnostics in cardiology: clinical interpretation]. Moscow: Prakticheskaya meditsina; 2009; 312 p.
- Nikitin N.P., Kliland D.D. Application of tissue myocardial Doppler echocardiography in cardiology. Kardiologia 2002; 3: 66–79.
- Alekhin M.N. Ul’trazvukovye metody otsenki deformatsii miokarda i ikh klinicheskoe znachenie [Ultrasound estimation techniques and their clinical significance]. Moscow: Vidar-M; 2012.
- Reznik E.V., Gendlin G.E., Storozhakov G.I. Ekhokardiografiya v praktike kardiologa [Echocardiography in cardiologist`s practice]. Moscow: Praktika; 2013; 212 p.
- Chan J., Hanekom L., Wong C., et al. Differentiation of subendocardial and transmural infarction using two-dimensional strain rate imaging to assess short-axis and long-axis myocardial function. J Am Coll Cardiol 2006; 48(10): 2026–2033, http://dx.doi.org/10.1016/j.jacc.2006.07.050.
- Takeuchi M., Borden W.B., Nakai H., et al. Reduced and delayed untwising of the left ventricle in patients with hypertension and left ventricular hypertrophy: a study using two-dimensional speckle tracking imaging. Eur Heart J 2007; 28(22): 2756–2762, http://dx.doi.org/10.1093/eurheartj/ehm440.
- Gjesdal O., Hopp E., Vartdal T., et al. Global longitudinal strain measured by two-dimension speckle tracking echocardiography is closely related to myocardial infarct size in chronic ischaemic heart disease. Clin Sci (Lond) 2007; 113(6): 287–296, http://dx.doi.org/10.1042/CS20070066.
- Roes S.D., Mollema S.A., Lamb H.J., et al. Validation of echocardiographic two-dimension speckle tracking longitudinal strain imaging for viability assessment in patients with chronic ischemic left ventricular dysfunction and comparison with contrast-enhanced magnetic resonance imaging. Am J Cardiol 2009; 104(3): 312–317, http://dx.doi.org/10.1016/j.amjcard.2009.03.040.
- Becker M., Hoffmann R., Kuhl H.P., et al. Analysis of myocardial deformation based on ultrasonic pixel tracking to determine transmurality in chronic myocardial infarction. Eur Heart J 2006; 27(21): 2560–2566, http://dx.doi.org/10.1093/eurheartj/ehl288.
- Choi J.O., Cho S.W., Song Y.B., et al. Longitudinal 2D strain at rest predicts the presence of left main and three vessel coronary artery disease in patients without regional wall motion abnormality. Eur J Echocardiogr 2009; 10(5): 695–701, http://dx.doi.org/10.1093/ejechocard/jep041.
- Park Y.H., Kang S.J., Song J.K., et al. Prognostic value of longitudinal strain after primary reperfusion therapy in patients with anterior-wall acute myocardial infarction. J Am Soc Echocardiogr 2008; 21(3): 262–267, http://dx.doi.org/10.1016/j.echo.2007.08.026.
- Abate E., Georgette E., Hoogslag M. Value of three-dimension speckle tracking longitudinal strain for predicting improvement of left ventricular function after acute myocardial infarction. J Am Cardiol 2012; 110(7): 961–967, http://dx.doi.org/10.1016/j.amjcard.2012.05.023.
- Toumanidis S.T., Kaladaridou A., Bramos D., et al. Apical rotation as an early indicator of left ventricular systolic dysfunction in acute anterior myocardial infarction: experimental study. Hellenic J Cardiol 2013; 54(4): 264–272.
- Pavlyukova E.N., Karpov R.S. Left ventricular deformity, rotation and axis rotation in coronary heart disease patients with severe left ventricular dysfunction. Terapevticeskij arhiv 2012; 9: 11–16.
- Jurcut R., Pappas C.J., Masci P.G., et al. Detection of regional myocardial dysfunction in patients with acute myocardial infarction using velocity vector imaging. J Am So of Echocardiogr 2008; 21(8): 879–886, http://dx.doi.org/10.1016/j.echo.2008.02.002.
- Kim H.-D., Kim H.-K., Kim M.-K., et al. Velocity vector imaging in the measurement of left ventricular twist mechanics: head-to-head one way comparison between speckle tracking echocardiography and velocity vector imaging. J Am Soc of Echocardiogr 2009; 22(12): 1344–1352, http://dx.doi.org/10.1016/j.echo.2009.09.002.
- Blutz T., Lang C.N., van Bracht M., et al. Segment-orientated analysis of two-dimensional strain and strain rate as assessed by velocity vector imaging in patients with acute myocardial infarction. Int J Med Sci 2011; 8(2):106–113, http://dx.doi.org/10.7150/ijms.8.106.
- Tkachenko S.B., Beresten' N.F. Tkanevoe doplerovskoe issledovanie miokarda [Tissue Doppler myocardial imaging]. Moscow: Real Taym; 2006; 176 p.
- Urheim S., Edvardsen T., Torp H., et al. Myocardial strain by Doppler echocardiography. Validation of a new method to quantify regional myocardial function. Circulation 2000; 102(10): 1158–1164, http://dx.doi.org/10.1161/01.CIR.102.10.1158.
- Connolly H.M., Oh J.K. Echocardiography. In: Libby P., Bonow R.O., Mann D.L., Zipes D.P. (eds.). Braunwald’s heart disease: a textbook of cardiovascular medicine. Chapter 14; Saunders; 2008; p. 227–314.
- Vasyuk Yu.A., Alekhin M.N., Khadzegova A.B., et al. Tkanevaya doppler-ekhokardiografiya i vektornyy analiz skorosti dvizheniya miokarda v otsenke funktsional’nogo sostoyaniya serdtsa [Tissue Doppler echocardiography and vector analysis of myocardial movement rate in heart functional state assessment]. Moscow: Anakharsis; 2007.
- Alekhin M.N. Tkanevoy doppler v klinicheskoy ekhokardiografii [Tissue Doppler in clinical echocardiography]. Moscow: 2005; 112 p.
- Pislaru C., Abraham T.P., Belohalavek M. Strain and strain rate echocardiography. Curr Opin Cardiol 2002; 17(5): 443–454.
- D'hooge J., Heimdal A., Jamal F., et al. Regional strain and strain rate measurements by cardiac ultrasound: principles, implementation and limitations. Eur J Echocardiogr 2000; 1(3): 154–170, http://dx.doi.org/10.1053/euje.2000.0031.
- Heimdal A., D′hooge J., Bijnens B., et al. In vitro validation of in-plane strain rate imaging. A new ultrasound technique for evaluating regional myocardial deformation based on tissue Doppler imaging. Echocardiography 1998; 15(8–II): 40.
- Henson R.E., Song S.K., Pastorek J.S., et al. Left ventricular torsion is equal in mice and humans. Am J Physiol Heart Circ Physiol 2000; 278(4): H1117– H1123.
- Opdahl A., Helle-Valle T., Remme E.W., et al. Apical rotation by speckle tracking echocardiography: a simplified bedside index of left ventricular twist. J Am Soc Echocardiogr 2008; 21: 1121–1128, http://dx.doi.org/10.1016/j.echo.2008.06.012.
- Torrent-Guasp F., Buckberg G.D., Clemente C., et al. The structure and function of the helical heart and its buttress wrapping. I. The normal macroscopic structure of the heart. Semin Thor Cardiovasc Surg 2001; 13(4): 301–319.
- Carasso Sh., Biaggi P., et al. Velocity vector imaging: standard tissue — tracking results acquired in normals — the VVI-STRAIN study. J Am Soc Echocardiogr 2012; 25(5): 543–552, http://dx.doi.org/10.1016/j.echo.2012.01.005.
- Solomon S.D., Anavekar N., Skali H., et al. Influence of ejection fraction on cardiovascular outcomes in a broad spectrum of heart failure patients. Circulation 2005; 112(24): 3738–3744, http://dx.doi.org/10.1161/CIRCULATIONAHA.105.561423.
- Han W., Xei M.X., Wang X.F., et al. Assessment of left ventricular torsion in patients with anterior wall myocardial infarction and after revascularization using speckle tracking imaging. Chin Med J (Engl) 2008; 212(16): 1543–1548.