Efficiency of Positron Emission Tomography with 18F-Fluorodeoxyglucose, 11С-Methionine and 82Rb-Chloride in Differential Diagnosis of Lung Tumors and Some Inflammatory Pulmonary Diseases
The aim of the investigation was to study the informativeness of positron emission tomography (PET) using 18F-FDG, 11С-methionine and 82Rb-chloride in differential diagnosis of tumor and some inflammatory pulmonary diseases.
Materials and Methods. PET findings of 378 patients with lung tumors and inflammatory pulmonary diseases were studied. PET with 18F-FDG and 11С-methionine were performed 120 and 15 min, respectively, after their intravenous administration. PET with 82Rb-chloride was performed 1 min after distant intravenous administration. Quantitative processing of PET findings regardless the medication used included visual image analysis and calculation of Standardized Uptake Value (SUV) in healthy pulmonary parenchyma and in lesion.
Results. SUV in patients with lung cancer in PET with 18F-FDG and 11С-methionine were higher than metabolic activity in an inflammation region, while in PET with 82Rb-chloride, SUV levels were significantly higher in the foci of inflammation than in malignant tumors. The patients with benign tumors and most patients with focal pneumofibrosis in pulmonary tissue consolidation area were recorded to have background distribution of radiopharmaceuticals. It enabled to reliably differentiate benign tumors and focal pneumofibrosis from lung cancer regardless the medications used.
Conclusion. The obtained data on the informativeness of positron emission tomography performed using 11С-methionine suggest high diagnostic value of the technique in the differential diagnosis of lung cancer, neuroendocrine tumors, benign tumors and inflammatory diseases. Despite good imaging potential PET with 82Rb-chloride is unreasonable in differentiation of lung tumors and inflammatory pulmonary diseases.
Currently, positron emission tomography (PET) is one of compulsory diagnostic procedures performed in patients with malignant tumors of any localization including pulmonary cancer (PC). It is due to both: high informational content of PET in most oncological diseases, and the introduction of new radioactive preparations (RP). These drugs are biological compounds labeled with positron-emitting radionuclides, which are able to accumulate in some morphological structures and reflect metabolic and dynamic processes. 18F-Fluorodeoxyglucose (18F-FDG) is currently the most extensively studied and widely used in oncology clinics RP of cyclotron production type. An indubitable advantage of this indicator is relatively large (110 min) half-life period that enables to scan in “the whole body” mode, as well as its capacity to accumulate in an increased amount in malignant tumors. 18F-FDG accumulation in malignant neoplasms is due to two main reasons: increase in the amount of transport proteins delivering RP in an atypical cell and activity rise of hexokinse catalyzing phosphorylation — the change from administered 18F-FDG to 18F-FDG-6-phosphate. The resulting molecule due to its large size and low activity of the following enzyme of glycolytic cascade — phosphohexoisomerase, and falls into a so-called metabolic trap, which promotes imaging of the most malignancies and their metastases as “hot” foci against healthy tissues. However, it is important to note that pathological hyperfixation of RP in pulmonary neoplasm is not always a radiological sign of a malignant process. Sometimes, 18F-FDG accumulation marks inflammatory changes [1–3]. It occurs, primarily, due to a significant increase of glucose transport proteins, as well as the accumulation of macrophages, neutrophils, eosinophils, granulocytes and other blood corpuscles, which are accumulator-cells, in the area of active inflammation. This property of 18F-FDG is certainly to restrain PET feasibility in differential diagnosis between PC and inflammatory diseases. In benign tumors, as well as in the area of cicatrical changes, 18F-FDG metabolic changes do not differ from glycolytic reactions in healthy pulmonary parenchyma. Accordingly, there is no an increased 18F-FDG scavenging in these diseases being a reliable differential diagnostic sign that makes it possible in PET with 18F-FDG to differentiate PC, benign tumors and local pulmonary fibrosis.
In literature there are few reports on using another RP in patients with PC — carbon 11 labeled methionine (11С-methionine) [4, 5]. It is important to note that over a number of years this indicator is used for differential diagnosis and treatment efficiency assessment of various brain tumors. However, 11С-methionine is rarely used to detect extracerebral tumors. This is due to the fact that in norm this RP accumulates mainly in internal secretion glands and excretory glands, as well as in bone marrow and spleen. It presents certain problems when estimating tumor extension in cancer patients. Moreover, a short half-life period of 11С (20 min), and, consequently, the problems arising from transporting of the indicator to other laboratories, prevent from its extensive use in isotope facilities with no cyclotron complex.
In its physicochemical properties methionine is a typical aliphatic sulfur-containing amino acid. In norm, after the agent enters blood flow, carrier proteins deliver it inside the cells, where mobile methyl group of amino acid is embedded into purine and pyrimidine bases of DNA molecule. When a normal cell transforms into a malignant one, as a rule, methylation is accompanied by numerous defects resulting in constant methionine deficiency. It is constant demand of atypical cells for methionine that leads to intensive uptake of amino acid exogenous fraction by different malignant tumors [6]. On the other hand, in literature there is information that 11С-methionine, like 18F-FDG accumulates not only in malignant tumors but also in inflammation area [4]. Despite this fact, in literature one can find reports on higher informativeness of 11С-methionine-PET in differentiation between PC and inflammatory diseases compared to 18F-FDG. However, it should be said that literature data on applying 11С-methionine-PET in patients with pulmonary masses of uncertain origin date mainly from the middle of the 90-ies and contain the information on the results of studies carried out in small groups of patients, who frequently had received anti-tumor treatment. In this regard, currently, the role of 11С-methionine-PET in the diagnosis of PC and inflammatory diseases needs elaboration.
Single foreign publications represent data on malignant tumor detection by PET with a generator radiotracer — rubidium-82 chloride (82Rb-chloride). It should be said that this radiotracer is used in cardiology to assess myocardial regional blood flow condition. Meanwhile, some authors performed 82Rb-chloride-PET in patients with cardiovascular pathology report about the accidental detection of malignant pulmonary tumors, breast cancer and other tumors fallen within scanning area [7–9]. Moreover, the researchers note adequate imaging of the masses detected. Currently, there are no reports both in foreign and Russian literature on unequivocal use of 82Rb-chloride in cancer patients.
82Rb-chloride is a normal saline containing nuclide ion 82Rb. By its physicochemical and biological properties ion 82Rb is an analogue of ion К+. A well known thallium-201 (201Tl) used for perfusion myocardial scintigraphy has similar characteristics. When injected intravenously, 82Rb-chloride, by analogy with 201Tl, is brought by blood flow to organs and tissues, where it is distributed in proportion to flow rate of regional capillary blood flow, as well as to the activity of sodium-potassium ATP-dependent pump. The mechanism of 82Rb-chloride uptake by malignant cells has not been identified yet. There has been only suggested that rubidium, by analogy with 201Tl, can come through membrane of atypical cells providing increased RP accumulation in a malignant tumor, and therefore, its imaging against healthy tissues as a “hot” focus [10].
The current study generalizes the experience of PET with the three above mentioned RP over the period 2011–2014 on the basis of Russian Research Center of Radiology and Surgical Technologies, and with direct participation of several pulmonary and oncology clinics in S. Petersburg.
The aim of the investigation was to study the informativeness ofpositron emission tomography using 18F-FDG, 11С-methionine and 82Rb-chloride in differential diagnosis of tumor and some inflammatory pulmonary diseases.
Materials and Methods. Chest PET with 18F-FDG, 82Rb-chloride and 11С-methionine was performed in 378 patients with lung tumors and inflammatory pulmonary diseases. In addition to PET, diagnostic examination of patients included bacteriological analysis of sputum or epithelial lining fluid, diaskintest, serological and polymerase chain reaction of blood plasma components to reveal antigens and DNA of Mycobacterium tuberculosis, as well as fibrobronchoscopy, radiography and computed tomography (CT) of chest. In 332 patients (87.8%) a final diagnosis was made relying on cytological and/or morphological analysis. The rest cases were assessed according to dynamic radiological control findings. Table 1 shows the classification of patients depending on a final diagnosis and RP used.
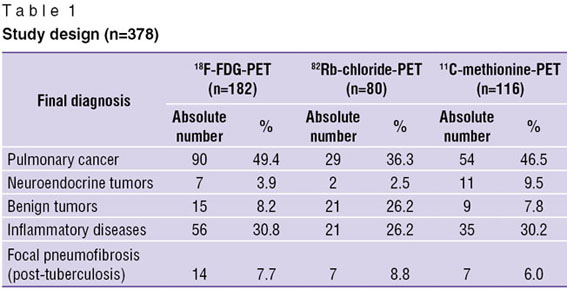 Table 1. Study design (n=378) Table 1. Study design (n=378)
|
The study complies with the declaration of Helsinki (adopted in June, 1964 (Helsinki, Finland) and revised in October, 2000 (Edinburg, Scotland)) and approved by the Ethics Committee of Russian Research Center of Radiology and Surgical Technologies. Written informed consent was obtained from all patients.
In accordance with a standard program, PET with 18F-FDG and 11С-methionine were performed 120 and 15 min, respectively, after their intravenous administration; 82Rb-chloride-PET was performed 1 min after distant intravenous administration. Quantitative processing of PET findings regardless RP used included visual image analysis and calculation of Standardized Uptake Value (SUV) in healthy pulmonary parenchyma and in lesion.
The findings were statistically processed using MedCalc 11.0.1 for Windows. In addition, we used parametric and nonparametric techniques including estimated mean (M), mean error (m). Critical significance level of a zero statistical hypothesis was taken equal to 0.05. Sensitivity, specificity, diagnostic accuracy, positive and negative prognostic value were determined using characteristic curve analysis (Receiver Operating Characteristic, ROC). In addition, SUV were cut-off values or numeral classifiers.
Results. Image analysis of tomograms obtained by using 18F-FDG showed pathological RP uptake in all patients with PC and the patients with inflammatory diseases. No increased accumulation of 18F–FDG was found in pulmonary benign tumors. No RP accumulation was also recorded in 3 out of 7 patients with neuro-endocrine tumors (NET) and in 7 out of 14 patients with post-tuberculosis pneumofibrosis.
82Rb-chloride-PET revealed foci of increased RP accumulation in the lung in 45 out of 80 patients. In 26 cases the changes were due to PC, in 2 cases — due to NET, in 17 — active tuberculosis. There was recorded no pathological 82Rb-chloride hyperfixation in all patients with benign pulmonary tumors and focal pneumofibrosis, in 3 PC patients, as well as in 4 cases of infiltrative tuberculosis.
Visual analysis of tomoscintigrams taken by chest scanning using 11С-methionine showed foci of pathological RP accumulation in all patients with PC and NET, in 31 out of 35 patients with inflammatory diseases, as well as in 2 out of 7 cases of focal pneumofibrosis. The rest patients were recorded to have background (consistent with intact pulmonary parenchyma) RP distribution in the tumor view. No signs of focal uptake were revealed.
Analysis of SUV recorded in patients with pulmonary tumors and inflammatory diseases detected by PET with focal RP uptake (Table 2) showed SUV recorded in PET with 18F-FDG and 11С-methionine to be significantly higher in patients with PC than in patients with inflammatory diseases and focal pneumofibrosis (p≤0.05). In addition, 82Rb-chloride-PET showed the opposite results: SUV above the inflammatory foci had significantly higher metabolic activity than those recorded in malignant pulmonary tumors (p=0.0012). 18F-FDG-PET in patients with NET demonstrated significantly lower SUV than in patients with PC (p=0.0026). However, SUV levels in NET patients did not differ from metabolic activity in foci of inflammation (p=0.1941). Similar findings were obtained in the study with 11С-methionine in patients with PC and NET. The comparison of SUV levels in these patients showed no significant differences (p=0.1341).
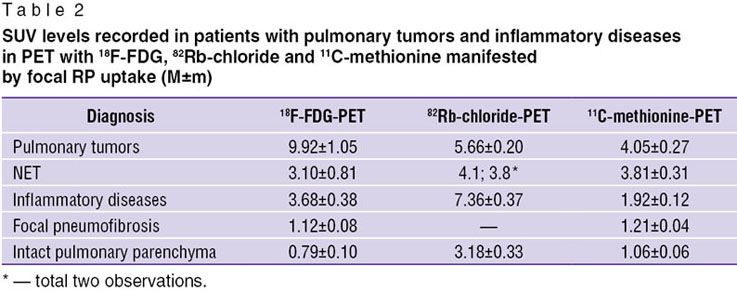 Table 2. SUV levels recorded in patients with pulmonary tumors and inflammatory diseases in PET with 18F-FDG, 82Rb-chloride and 11С-methionine manifested by focal RP uptake (M±m) Table 2. SUV levels recorded in patients with pulmonary tumors and inflammatory diseases in PET with 18F-FDG, 82Rb-chloride and 11С-methionine manifested by focal RP uptake (M±m)
|
Thus, imaging findings, as well as the results of comparing mean SUV calculated in PET with 18F-FDG, 11С-methionine and 82Rb-chloride indicated similar semiotic manifestations and quantitative characteristics in patients with pulmonary tumors and pulmonary inflammatory diseases. In this regard, we performed ROC analysis to determine numerical differential diagnostic criteria, as well as to calculate PET informativeness. Threshold SUV and informativeness of PET with 18F-FDG, 82Rb-chloride and 11С-methionine in differential diagnosis of pulmonary tumors and inflammatory diseases (Table 3) indicate that maximum values of diagnostic accuracy of PET in differential diagnosis of pulmonary tumor and inflammatory diseases were found in 11С-methionine-PET. And specificity values in 18F-FDG and 11С-methionine-PET were comparable. Informativeness indices in differentiation of malignant and inflammatory changes in the lungs using 82Rb-chloride-PET were significantly lower than those obtained in 18F-FDG and 11С-methionine-PET.
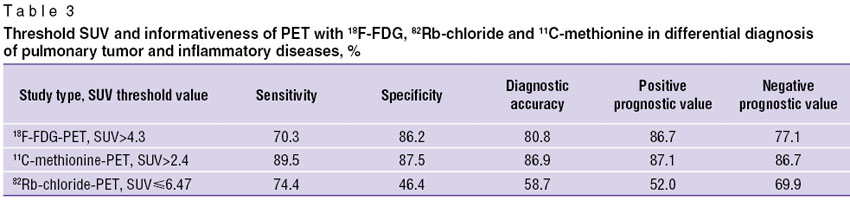
|
Table 3. Threshold SUV and informativeness of PET with 18F-FDG, 82Rb-chloride and 11С-methionine in differential diagnosis of pulmonary tumor and inflammatory diseases, % |
Discussion. Currently, 18F-FDG-PET is widely used in patients with pulmonary masses of uncertain origin. The main advantage of the technique is the possibility to perform differential diagnosis of malignant and benign pulmonary tumors, as well as determine the stage of a tumor process in patients with verified PC. However, despite a long-term experience of using 18F-FDG-PET in such patients, it would be incorrect to consider the problem of differentiation of PC and inflammatory disease to be resolved. Unfortunately, 18F-FDG-PET shows malignant and inflammatory processes in the ling as equally increased RP accumulation. So, the search and introduction of more specific radiotracers are required.
For that purpose we compared informativeness of PET performed with a widely used 18F-FDG with the efficiency of the method using other tracers: 11С-methionine and 82Rb-chloride. The results of visual analysis of PET with 18F-FDG, 11С-methionine and 82Rb-chloride indicated similar semiotic manifestations of pulmonary tumor and inflammatory diseases. In patients with benign tumors and the most patients with focal pneumofibrosis there was recorded background RP distribution in the pulmonary tissue consolidation area. It enabled to classify reliably benign tumors and focal pneumofibrosis from PC regardless of RP used. However, in PET with 18F-FDG, 11С-methionine and 82Rb-chloride the most patients with inflammatory diseases, as well as the patients with malignant pulmonary tumors had RP accumulation of different intensity degree in the tumor area. The comparison of SUV levels in patients with PC and inflammatory diseases revealed significant differences between metabolic activity indices using all the tracers under study. SUV were higher than metabolic activity in the inflammation area in PET with 18F-FDG and 11С-methionine in PC patients. On 82Rb-chloride-PET, SUV levels in inflammatory foci were significantly higher than in malignant tumors. In addition, despite the stated significant differences between SUV levels in patients with PC and inflammatory diseases, and good imaging of pathological masses on 82Rb-chloride-PET, informativeness indices indicated inadequate differential and diagnostic properties of this radiotracer.
The results of 18F-FDG-PET and 11С-methionine-PET comparison appeared to be more satisfactory. On 18F-FDG-PET in 3 out of 7 patients with typical pulmonary carcinoids there were recorded no increased accumulation of RP in tumor, i.e. we received false-negative results. Moreover, on 11С-methionine-PET in all patients with NET, RP accumulation was observed in pathological mass of the lung that helped to diagnose a malignant tumor. In addition, on 11С-methionine-PET compared to 18F-FDG-PET in 4 tuberculosis patients the process in the lung was correctly estimated as inflammatory changes due to the lack of increased RP accumulation in the tumor area. ROC-analysis of 11С-methionine-PET findings enabled to state that the highest informativeness indices in differentiation between pulmonary tumors and pulmonary inflammatory diseases were recorded when using this radiotracer.
Conclusion. The findings on informativeness of positron emission tomography using 11С-methionine suggest high diagnostic accuracy of the technique in differential diagnosis of pulmonary cancer, neuro-endocrine tumors, benign tumors and inflammatory diseases. 82Rb-chloride-PET in spite of good imaging possibilities is unreasonable to use for differentiating pulmonary tumors from pulmonary inflammatory diseases.
Study Funding and Conflict of Interests. The study was not supported by any funds, and the authors have no conflict of interest to disclose.
References
- Tlostanova M.S., Avetisyan A.O. The informativeness of positron emission tomography with [18F]-fluorodeoxyglucose in differential diagnosis of lung cancer. Vestnik RGMU 2012; 2: 41–44.
- Tlostanova M.S., Yablonsky P.K., Pishchik V.G., Levchenko E.V., Avetisyan A.O., Petrov A.S. The new approaches to quantitative analysis of positron emission tomography with [18F]-fluorodeoxyglucose data in patients with various diseases of the bronchopulmonary system. Vestnik RGMU 2012; 6: 45–48.
- Kumar R., Halanaik D., Malhotra A. Clinical applications of positron emission tomography — computed tomography in oncology. Indian J Cancer 2010; 47(2): 100–119, http://dx.doi.org/10.4103/0019-509X.62997.
- Hsieh H.J., Lin S.H., Lin K.H., Lee C.Y., Chang C.P., Wang S.J. The feasibility of 11C-methionine-PET in diagnosis of solitary lung nodules/masses when compared with 18F-FDG-PET. Ann Nucl Med 2008 22(6): 533–538, http://dx.doi.org/10.1007/s12149-007-0142-8.
- Zhao S., Kuge Y., Kohanawa M., Takahashi T., Zhao Y., Yi M. Usefulness of 11C-methionine for differentiating tumors from granulomas in experimental rat models: a comparison with 18F-FDG and 18F-FLT. J Nucl Med 2008 49(6): 135–141, http://dx.doi.org/10.2967/jnumed.107.044578.
- Mattoli M.V., Treglia G., Trevisi G., Muoio B., Cason E. Usefulness of 11C-methionine positron emission tomography in differential diagnosis between recurrent tumours and radiation necrosis in patients with glioma: an overview. The Open Neurosurgery Journal 2012 (5): 8–11.
- Mirpour S., Khandani A.H. Extracardiac abnormalities on rubidium-82 cardiac positron emission tomography/computed tomography. Nucl Med Commun 2011 Apr; 32(4): 260–264, http://dx.doi.org/10.1097/MNM.0b013e3283440dcb.
- Khandani A., Sheikh A., Beavers G., et al. Extra-cardiac findings on PET portion of Rubidium-82 (Rb-82) cardic PET-KT. J Nucl Med 2010 51(Suppl 2): 1018.
- Gupta A., DiFilippo F.P., Brunken R.C., et al. Rubidium-82 uptake in metastases from pheochromocytoma on PET myocardial perfusion images. Clin Nucl Med 2011 36(10): 930–931, http://dx.doi.org/10.1097/RLU.0b013e31822920b7.
- Hisada K., Tonami N., Miyamea T., et al. Clinical evaluation of tumour imaging with 201Tl chloride. Radiology 1978 129: 497–500.










