Diagnostic Ultrasound of Diffuse-Nodular Pleural Mesotheliomas — Echosemiotics, Growth Characteristics, and Scanning Techniques
The aim of the investigation was the optimization of chest ultrasound technique in the diagnosis of diffuse-nodular pleural mesotheliomas, development and systematization of their ultrasound semiotics.
Materials and Methods. We studied the ultrasonic picture of 32 mesotheliomas (31 malignant), among them 30 — diffuse-nodular, 2 — focal.
Results. There were developed the assessment criteria of diffuse-nodular mesothelioma echosemiotics: significant (over 15 mm) diffuse thickening of costal and diaphragmal pleura, with homogeneous hypoechoic structure and sharp contour against a marked (700–1500 ml) anechoic pleural effusion. For the first time we revealed the predominance of anterior costal and diaphragmal pleural lesions with typical caudal extension of the whole phrenicocostal sinus outwards. Chest ultrasonography optimization in mesothelioma diagnosis consists in longitudinal chest scanning from parasternal to anterior axillary line, first in V–VII intercostals space and then under costal arch with the following probe displacement down the anterior abdominal wall to assess the tumor extension in soft tissues. Typical deformity and diaphragm thinning resulted from tumor extension into diaphragm were described.
Conclusion. Chest ultrasonography is a high-quality diagnostic technique of diffuse-nodular mesothelioma enabling to determine tumor malignance, its extension, size, and invasion in surrounding tissues.
Mesothelioma is a relatively rare malignancy arising from multipotent mesothelial cells and characterized by the involvement of all parts of parietal and then — visceral pleura, mainly with diffuse growth on pleural layers [1]. Chest survey radiograph enables only to suspect mesothelioma, the basic diagnostic and staging technique being computed tomography. Despite the advances in computed tomography expansion, in some cases its accessibility is still rather limited. Therefore, chest ultrasonography is extensively used as an alternative and additional diagnostic technique [2, 3]. One of promising echographic directions is the detection and differential diagnosis of tumor and inflammatory pleural changes.
The principal echo-criteria of mesothelioma are presented in literature but without detailed description and information, mainly, due to a few observations and general approach to noninvasive diagnostics with an advantage given to puncture biopsy [4, 5]. There have been researches suggesting differential and diagnostic criteria of pleural effusions of different etiology including those in mesotheliomas [6, 7]. However, ultrasound semiotics of mesotheliomas and their imaging technology considering their growth type, localization and dissemination are understudied, and a detailed analysis based on sufficient material is required to develop a united diagnostic approach.
The aim of the investigation was the optimization of chest ultrasound technique in the diagnosis of diffuse-nodular pleural mesotheliomas, development and systematization of their ultrasound semiotics.
Materials and Methods. The study is based on chest ultrasonography findings of 32 patients with pleural mesotheliomas who had undergone medical treatment in Nizhny Novgorod Clinical Hospital No.5 in 2004–2013, among them 17 male (53%) and 15 female (47%) patients, mean age — 58.7 years.
The study complies with the declaration of Helsinki (adopted in June, 1964 (Helsinki, Finland) and revised in October, 2000 (Edinburg, Scotland)) and approved by the Ethics Committee of Nizhny Novgorod State Medical Academy. Written informed consent was obtained from all patients.
Ultrasound investigation was performed on an ultrasound scanner (ESAOTE, Italy) of middle class by mechanical sector probes 3.5 and 7.5 MHz, and recorded on a video tape recorder with the subsequent computer digitizing.
Pleura and pleural cavity were ultrasonically examined in patients in plantigrade position from intercostals space in longitudinal and transverse scan planes according to a developed technique [8] with sequential examination of the posterior, lateral and anterior thoracic surface and an obligatory examination of the anterior phrenicocostal sinuses and the adjacent diaphragmatic parts. The patients were examined on free breathing and breath holding in the phase of full inspiration or expiration. If necessary, a forced expiration test was used; for the test a probe was placed above the contact area of a parietal mass with the thoracic wall perpendicular to the body surface, and in slow deep breathing we assessed the object`s mobility relating to the surface of the lung and the ribs.
If any pleural effusion, we studied all pleural layers including mediastinal concerning masses with infiltrative or focal growth. We assessed the extension of the involvement, medium and maximum thickness of the lesion and the thickness uniformity along the length of the mass, the characteristics of the contours, echogenicity of pathological tissue and its relationship with the surrounding structures.
All studies were verified by histological findings of surgical or puncture material, cytological and microbiological analysis of sputum, punctuates of pleural cavity or subpleural masses, bronchial washing in bronchoscopy, the findings of comprehensive clinicoradiological investigation.
Results and Discussion. Nearly all 32 mesotheliomas (31, 97%) were malignant, and only one — benign. 30 patients (94%) were found to have a diffuse-nodular form, 2 (6%) — focal, one of them — benign tumor. The right involvement was more frequent than the left one — 24 (75%) and 8 (25%), respectively. In most cases ultrasound detection of tumors were incidental findings, when the examined patients had an X-ray pleural effusion pattern.
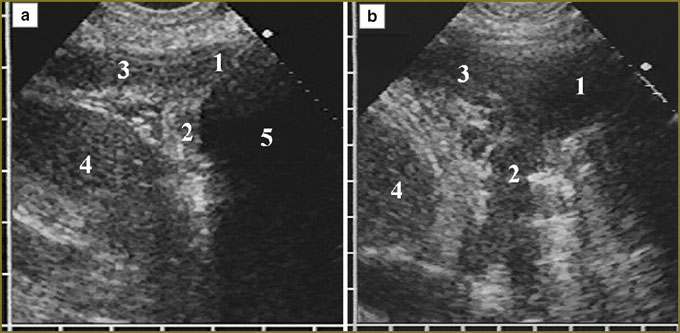
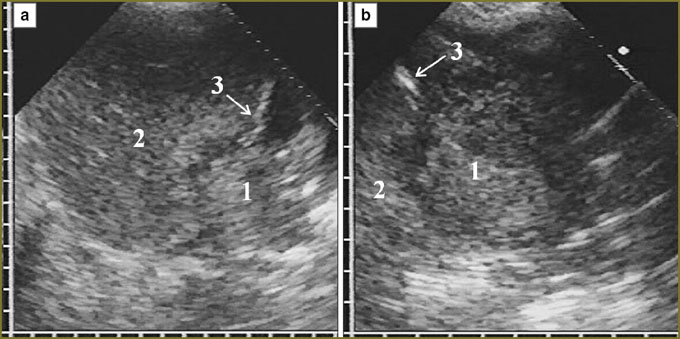
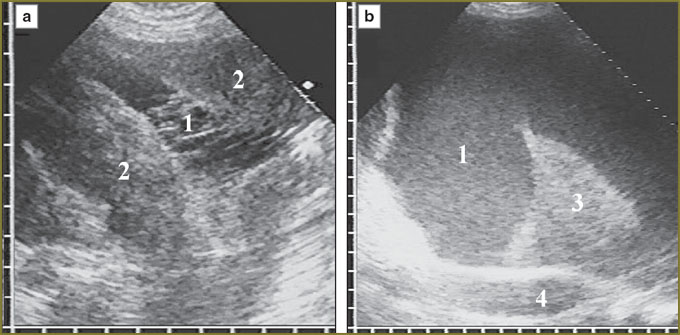
At the moment of detection, all 30 diffuse-nodular mesotheliomas were disseminated and located in the form of a diffuse thickening, over 10 mm, of at least one pleural layer extending over 10 cm along the thoracic wall. Further, the echo-picture of diffuse-nodular mesothelioma was analyzed based on the present 30 observations taken for 100%. In 2 (6%) patients the tumor involved costal pleura alone, in 23 (77%) — costal and diaphragmatic pleura, in 5 (17%) — costal, diaphragmatic and visceral pleura. There were mainly affected the anterior parts of costal and diaphragmatic pleura (21 patients, 70%) with the anterior phrenicocostal sinus involved due to the tumor penetration into the sinus at various depth (Fig. 1).
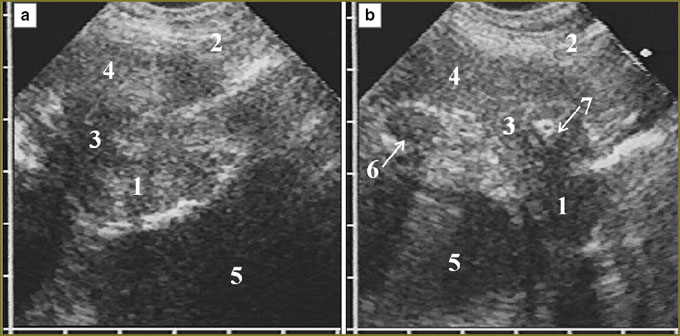
In 11 patients (37%) a tumor occupied the entire anterior phrenicocostal sinus and extended downward, beyond the sinus, having the form of an unmoved in inspiration linear-oval mass with hypoechoic structure, which situated in front of the liver or the spleen. The tumor tissue had a clear smooth contour, well-delimitated from the presenting soft tissues of the anterior abdominal wall. Inside it was bounded by a thin immobile echogenic line of parietal peritoneum, liver covered by echogenic capsule being adjacent to it (Fig. 2). In some patients these serous layers were imaged integrally and the capsule could be differentiated only in forced respiration by its dislocation with the liver towards the abdominal wall.
For the first time in clinical practice we described typical tumor localization with phrenicocostal sinus full-length invasion and further caudal extension beyond the sinus. Such an echo-picture we interpret as the sign of local extension of the tumor due to the invasion in soft tissues of the anterior abdominal wall. For such diagnostics, target examination of the anterior pleural recess in chest ultrasonography is necessary; it requires longitudinal scanning in V–VII intercostal space from parasternal to anterior axillary line, and against pleural effusion detect diffuse thickening of diaphragmatic and costal pleura with the invasion in the anterior phrenicocostal sinus. A probe is to be placed under the costal arch and relocated down the anterior abdominal wall making a series of longitudinal scans along the mentioned anatomical topographic lines, following mesothelioma extension deep in the anterior phrenicocostal sinus.
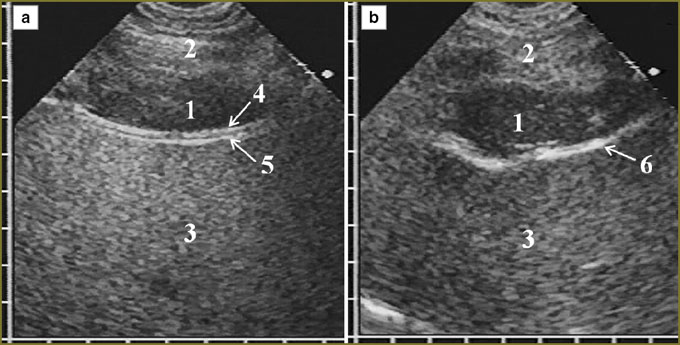
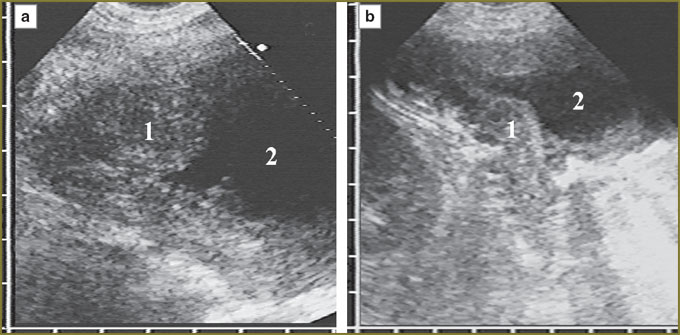
Tumor tissue invasion of the anterior phrenicocostal sinus was accompanied by its dilatation in anterioposterior direction. If the tumor was up to 15 mm in thickness, it had no effect on the shape of the anterior hepatic surface, but 20-mm thickness resulted in the hepatic contour deformity along the depressed arch in the area of contact with mesothelioma. Deformity grew with tumor thickness increase. If there was no tumor tissue invasion in the parietal peritoneum and the liver capsule, their hyperechoic signal was clearly traced in the form of a continuous line rounding the medial border of the tumor, the liver respiratory mobility being preserved. We could judge of mesothelioma invasion from the anterior abdominal wall into the liver by the disappearance of a capsule image with normal liver parenchyma substituted by tumor tissue in the contact area of the liver and the anterior abdominal wall, there being no the liver respiratory mobility (Fig. 3).
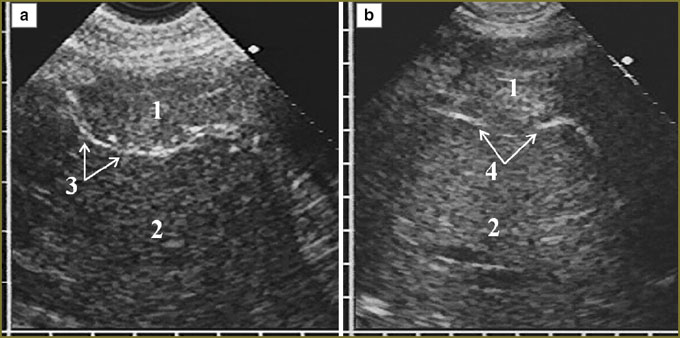
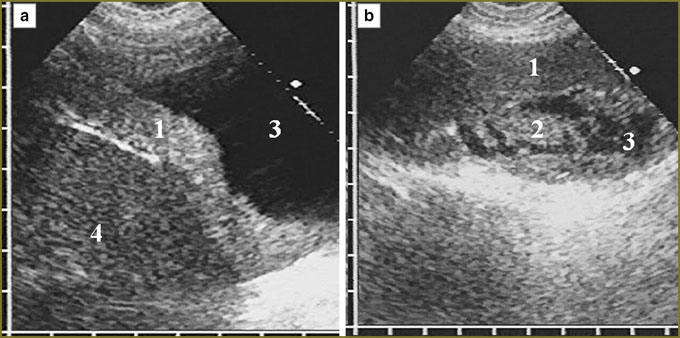
In mesothelioma invasion in the diaphragm superiorly there was significant deformity of the organ in the form of folds and protrusions towards the thoracic cavity and located against the surrounding tumor, as well as irregular local thinning of the cupola in tumor tissue invasion in it with partial displacement of normal echostructure (Fig. 4). There was no respiratory mobility of the diaphragm in all cases of diffuse thickening (over 20 mm) of the pleura and in the extension of tumor through the sinus to costal pleura.
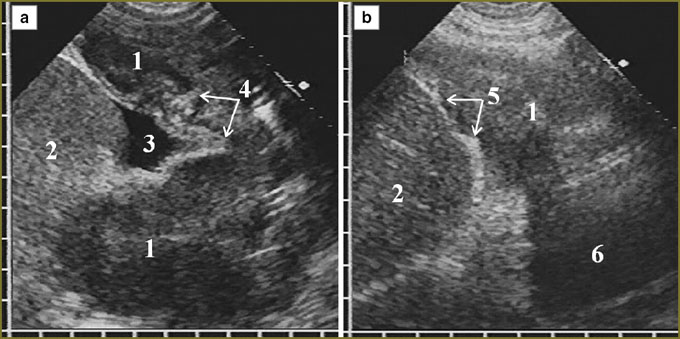
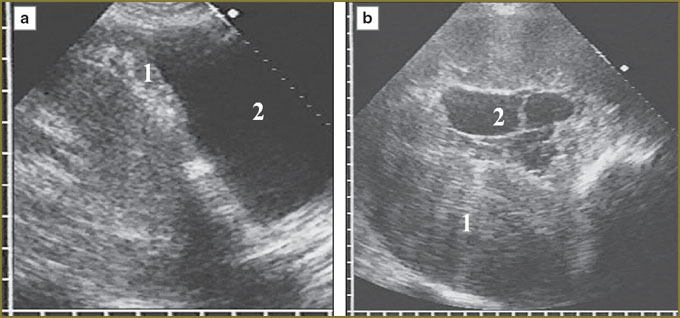
One patient with a large diffuse-nodular mesothelioma occupying mainly the posterior parts of diaphragmatic pleura had extensive tumor invasion in the liver, diaphragm echo signals disappearing for 4.5 cm with tumor tissue invasion into the liver up to 3.5 cm deep (Fig. 5). In nodular mesothelioma extending from costal pleura we diagnosed deep invasion in soft tissue of the thoracic wall with two intercostal spaces involved, cuff accretion and partial destruction of IV costal cartilage (Fig. 6).
|
|
Nearly in all patients diffuse-nodular mesothelioma had a layer shape of irregular thickness, extending along pleural layers for various distances. 3 patients (10%) including one with the recurrence of the disease additionally had a marked nodular tumor component located in the form of heterogeneous parietal foci and echogenic masses of different sizes, irregular-shaped, with extremely irregular contours (Fig. 7). Mesothelioma always had clear contours, which could be both: regular and irregular, wavelike. In patients without pleural effusion an irregular boundary with air pulmonary tissue on distant tumor areas could be due to the invasion into the lung (Fig. 8).
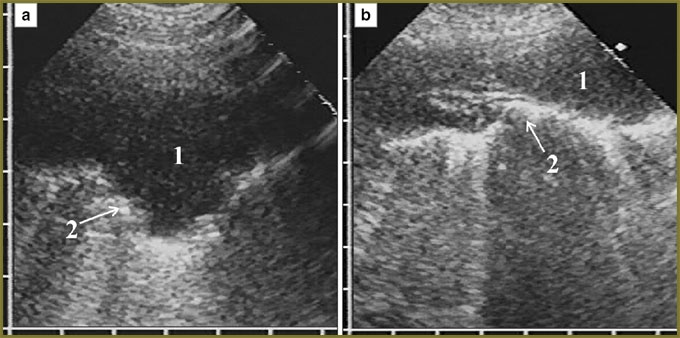 Fig. 8. Mesothelioa without pleural effusion, with non-uniform thickness and irregular boundary with the lung (а, b): mesothelioma (1), irregular hyperechoic surface of the lung (2) Fig. 8. Mesothelioa without pleural effusion, with non-uniform thickness and irregular boundary with the lung (а, b): mesothelioma (1), irregular hyperechoic surface of the lung (2)
|
All tumors had homogeneous structure and low echogenicity, slightly higher or lower than the echogenicity of normal liver and spleen, or comparable with it (See Fig. 2). There were found no relationships between tumor echogenicity, thickness, extension, localization and the presence of pleural effusion.
In most patients (n=19, 63%) diffuse nodular mesothelioma had maximum thickness within the range of 15–30 mm, relatively thick tumor up to 15 mm was revealed in 5 patients (17%), and in 6 patients (20%) tumor thickness exceeded 30 mm, moreover, in three of them the tumor occupied significant volume of the pleural cavity, its maximum thickness being 60–80 mm (Fig. 9). In 9 patients (30%) mesothelioma had rather uniform thickness, and the difference between its maximum and minimum did not exceed 3–4 mm, 21 patients (70.0%) were found to have non-uniform thickness of the diffuse component of the tumor, thickness variation being over 5 mm.
|
|
Associated exudative pleuritis was found in 29 of 32 patients (91%); in the rest 3 patients (9%) mesothelioma was not accompanied by fluid accumulation in the pleural cavity. Pleural effusion volume varied from 50 ml, which was removed by puncture, to 2.5 liters of evacuated fluid a day (Fig. 10). Inmostpatients (24, 83%) thevolumeofsingle-stageevacuatedfluidwas 800–1500 ml. No correlation between the effusion volume and tumor extension and size was found: small amount of fluid up to 100 ml was obtained in both: in a relatively small mesothelioma on costal pleura alone, and also in massive tumor invasion of all pleural layers with diaphragm involvement. As a rule, effusion volume about a liter was found in patients with diffuse involvement of costal and diaphragmatic pleura.
In accordance with puncture or drainage findings, evacuated fluid was frequently (18 patients, 62%) serous, straw-colored, clear or slightly cloudy, 11 patients (38%) had serosanguineous or hemorrhagic exudate. Echographically anechoic, acoustically clear fluid was found in 20 patients (70%). Fine-grained homogeneous dense mobile echogenic suspension was located in effusion of 4 patients (14%) (Fig. 10, b). According to the echo-picture, the suspension was consistent with hemorrhagic effusion, in three of four patients there was obtained hemorrhagic exudate, in one — cloudy serosanguineous fluid. In 5 patients (17%) the suspension was non-specific — fine- and medium-grained, not entirely homogeneous, and relatively rare, due to what we could not definitely characterize it as hemorrhagic, however, in three of them the effusion appeared to be serosanguineous. In general, the comparison results prove the previous data [6] on possible hemorrhagic nature of the effusion in case it contains fine-grained homogeneous dense mobile suspension.
Fibrin fibers in the effusion were found in 7 patients (24%) with significant tumor process extension in pleura (Fig. 10, а). In 4 of them a massive mesothelioma involved all 3 pleural layers and had maximum — in our study — sizes, the effusion volume not exceeding 500 ml. The fibers were not numerous, thin, crossed the effusion in the form of single septa, and only occasionally they formed parietal areas with honeycombed structure.
According to thoracic ultrasonography, there were two cases of mesothelioma hyperdiagnosis, mesothelioma diagnosis was not histologically confirmed, and the cases were not included in the study group (Fig. 11). In one case the tumor process was imitated by massive pleural thickenings after previous exudative pleuritis (2 months prior to thoracic ultrasonography), concerning which the patient underwent hospital treatment and was examined in the thoracic surgery department due to the revealed opacity in the low lung field on the right. In the second case an extensive metastatic process in pleura (subsequently diagnosed as gastric cancer) was taken for mesothelioma.
The only benign nodular mesothelioma was assessed preoperatively as intercostal neurinoma, and within 2 years it was followed up (Fig. 12). Over the following up period it grew slowly, the size increasing from 47´27 mm to 57´37 mm. The final diagnosis was made with the help of histological investigation of the removed tumor specimen.
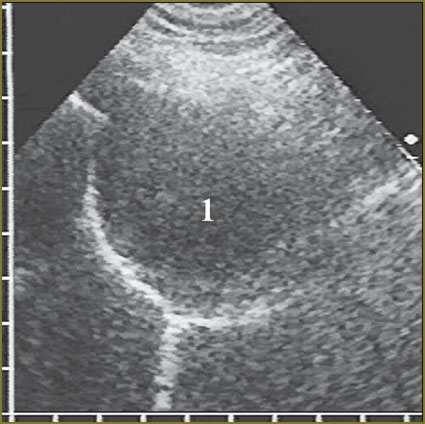 Fig. 12. Benign nodular mesothelioma (1) imitating intercostal neurinoma Fig. 12. Benign nodular mesothelioma (1) imitating intercostal neurinoma
|
Thus, typical echosemiotics is large non-uniform thickening of at least two pleural layers, more frequently — costal and diaphragmatic, with clear regular contour and homogeneous hypoechoic structure revealed against the marked anechoic pleural effusion. The characteristic feature is the tumor invasion throughout the anterior phrenicocostal sinus of pleura with the following caudal extension beyond the sinus that requires a target examination of this area. For his purpose longitudinal scanning of the chest from parasternal to the anterior axillary line is performed with the following displacement of a probe down the anterior abdominal wall.
Conclusion. Chest ultrasonography is a high-quality radiologic safe diagnostic technique of diffuse-nodular mesothelioma enabling to determine tumor malignance, its extension, size, invasion in surrounding tissues, and if necessary — to find an optimal place for puncture biopsy.
Study Funding and Conflict of Interests. The study was not supported by any funds, and there are no conflict of interest related to the study.
References
- Yablonskiy P.K., Petrov A.S. Malignant pleural mesothelioma. Prakticheskaya onkologiya 2006; 3: 179–188.
- Sinyukova G.T., Sholokhov V.N., Gudilina E.A. Diagnostic ultrasound of pleural masses. Ul’trazvukovaya diagnostika 2000; 1: 98–101.
- Beckh S., Blcskei P.L., Lessnau K.D. Real-time chest ultrasonography: a comprehensive review for the pulmonologist. Chest 2002; 122(5): 1759–1773, http://dx.doi.org/10.1378/chest.122.5.1759.
- Reuβ J. Sonographie der pleura. Ultraschall Med 2010; 31(1): 8–25, http://dx.doi.org/10.1055/s-0028-1109995.
- Mathis G. Chest sonography. Berlin, Heidelberg, New York: Springer Verlag; 2008; 242 р.
- Safonov D.V., Shakhov B.E. Ul‘trazvukovaya diagnostika plevral‘nykh vypotov [Diagnostic ultrasound of pleural effusions]. Moscow: Vidar-M; 2011; 104 p.
- Abouzgheib W., Bartter T., Dagher H., et al. A prospective study of the volume of pleural fluid required for accurate diagnosis of malignant pleural effusion. Chest 2009; 135; 999–1001, http://dx.doi.org/10.1378/chest.08-2002.
- Safonov D.V., Shakhov B.E. Ul‘trazvukovaya diagnostika vospalitel‘nykh zabolevaniy legkikh [Ultrasonic diagnosis of pulmonary inflammatory diseases]. Moscow: Vidar-M; 2011; 120 p.