Compression Elastography in Endosonography as an Early Differential Diagnostic Technique of Liver Fibrosis Stages
The aim of the investigation was to study the capabilities of compression elastography used in endosonography to estimate the stage of fibrosis process in liver parenchyma.
Materials and Methods. We examined 67 patients with hepatic diseases including 29 patients (43.3%) with steatosis, 23 (34.3%) — with hepatitis, 15 (22.4%) — with cirrhosis. All patients underwent hepatic sonography, transient elastography, fibrogastroduodenoscopy, compression elastography in the course of endosonography. Ultrasound-assisted liver puncture was used as a reference method.
Results. The survey enabled to determine optimal threshold levels of compression elastography in steatosis, hepatitis, liver cirrhosis, when diagnostic sensitivity, specificity and accuracy of the technique are maximal. The diagnosis of hepatic steatosis is confirmed if difference coefficient is from 5 to 17 RU (F0-stage) and from 18 to 25 RU (F1-stage), hepatitis — from 26 to 37 RU (F2-stage) and from 38 to 49 RU (F3-stage), liver cirrhosis — from 50 to 100 RU (F4-stage).
Conclusion. Compression elastography in endosonography enables to image hepatic segments (segments 1, 2, 4, 5, 8), which are not always accessible in transabdominal sonography, and are inaccessible in transient elastometry; therefore, making it possible to improve early diagnostics of liver fibrosis process.
Transabdominal ultrasound and transient elastometry do not show early liver parenchyma changes in less accessible hepatic segments (1, 2, 4, 5, 8), and by that they cannot verify malignant and benign pathology [1–4]. In obese patients, with narrow intercostals space, it presents a problem to place sensors, and reduces the correctness of findings [5–12].
Needle biopsy remains a gold standard of liver disease diagnosis [13–17]. However, the technique has a number of disadvantages: invasiveness, low tolerability by patients, contradictions to the manipulation, low sensitivity, since the volume of the tissue under study is just about 1/50 000 of an organ. Moreover, insufficient experience of medical staff in liver biopsy, and in the interpretation of morphological data of a bioptate can contribute to inaccuracy of findings [17–19].
The main selection criteria for an instrumental diagnostic technique are informativeness, accessibility, potential danger, price-efficiency ratio [9–11, 18–21]. An adequate and early assessment of pathological process intensity in liver parenchyma will enable to determine a disease stage, make a diagnosis, and timely correct the patients` management [22–24]. Currently, one of promising diagnostic methods of early hepatic fibrosis is hepatic elastography [9, 10, 16, 17]. Technological background of the technique use is clinical experience in the interpretation of liver consolidation in palpation towards marked fibrosis or hepatic cirrhosis [14, 17, 21–23, 25–27]. Elastographic indices show the intensity degree of hepatic fibrosis, however, to improve the diagnostic value of non-invasive methods it seems appropriate to combine them in order to increase sensitivity and specificity [13–16, 19, 21, 25, 27].
The use of compression elastography during hepatic endosonography will enable to prognosticate fibrosis process development more accurate, and assess its extension. This is particular significant when a patient cannot undergo liver needle biopsy or there is a focal hepatic fibrosis, which is inaccessible for transient elastography due to technical impossibility of performance.
The aim of the investigation was to study the capabilities of compression elastography used in endosonography to estimate the stage of fibrosis process in liver parenchyma.
Materials and Methods. The study was carried out in Smolensk Clinical Hospital No.1. We examined 67 patients with hepatic diseases, the clinical forms being the following: steatosis — 29 (43.3%), hepatitis — 23 (34.3%), cirrhosis — 15 (22.4%). All patients underwent hepatic sonography, transient elastography, fibrogastroduodenoscopy and compression elastography in the course of endosonography by one doctor, in one room. Ultrasound-assisted liver puncture was used as a reference method. Biopsy material was taken in 40 patients: 22 patients (55%) with steatosis, 15 (37.5%) — with hepatitis, 3 (7.5%) — with hepatic cirrhosis.
All patients underwent complex diagnostic ultrasound of abdominal cavity with Doppler and portal blood flow assessment on HITACHI Preirus (Japan), in duplex and triplex scanning modes. Endosonography with elastography was performed using a ultrasonograph and an endoscope with convex probe PENTAX EG 387OUTK (Japan), transient elastometry — on Fibroscan (Echosens, France).
Liver compression elastography was performed concurrently with endosonography, successively placing a convex probe in cirrhotic changes in views of the segments 1, 2, 4, 5, 8, as follows: in descending part, duodenal bulb, antral part, and the body of stomach. We determined difference coefficients of compression elastography strain ratio (SR) (RU).
Results and Discussion. After transient elastometry, compression elastography in endosonography and liver biopsy in all clinical forms we compared their findings (Table 1).
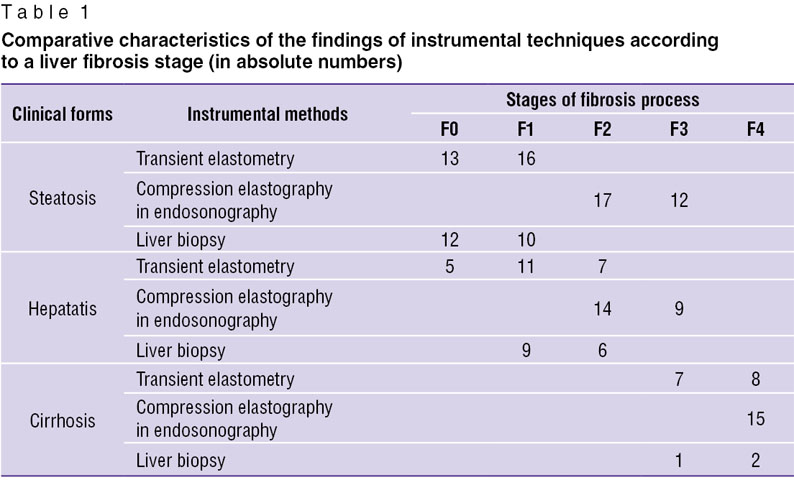 Table 1. Comparative characteristics of the findings of instrumental techniques according to a liver fibrosis stage (in absolute numbers) Table 1. Comparative characteristics of the findings of instrumental techniques according to a liver fibrosis stage (in absolute numbers)
|
Compression elastography in endosonography was two-staged: qualitative assessment of elastographic view of liver parts and a corrective stage of elastographic diagnosis — measurement of elastographic correlation in revealed regions of interest by calculating comparative difference coefficients of strain ratio (SR).
High-quality color images in compression elastography during endosonography causes certain difficulties due to artifacts, which were caused by echoendoscope rotation for more accurate positioning of a convex probe and by digestive tube contractions that required patient’s position changing (prone position).
The color characteristics in tissue image evaluation program have three main colors: red, green and blue indicating stiffness degree. In liver compression elastography, i.e., in fibrosis stage assessment, we obtained the following characteristics: mixed tissue staining (green + red + blue) was typical for F0, F1, F2 stages, hard staining (blue color prevailing) –– for F3 and F4 stages.
If SR difference coefficient was from 5 to 17 RU (F0-stage) and from 18 to 25 RU (F1-stage) hepatic steatosis was confirmed, from 26 to 37 RU (F2-stage) and from 38 to 49 RU (F3-stage) — hepatitis, from 50 to 100 RU (F4-stage) — hepatic cirrhosis.
Hepatic compression elastography values demonstrated statistically significant difference of tissue SR in different fibrosis stages. Liver parenchyma stiffness can be compared only in complex elastography (transient elastometry + compression elastography), since liver fibrous process develops irregularly, and the use of one of elastographic techniques do not enable to present an entire pattern of liver fibrous process, particularly in case of contradictions to biopsy.
Comparing liver biopsy and transient elastometry findings using Kolmogorov–Smirnov test (d=0.33125; p<0.01), Lilliefors test (p<0.01) and Shapiro–Wilk test (SW–W=0.68460; p=0.0001) we found that the hypothesis for Gaussian distribution model is applicable, the sign having normal distribution (p=0.0001).
For compression elastography in endosonography Kolmogorov–Smirnov test (d=0.32424; p<0.01), Lilliefors test (p<0.0100) and Shapiro–Wilk test (SW–W=0.69076; p=0.0001) also showed that the hypothesis for Gaussian distribution model was applicable, SR difference coefficient had normal distribution (p=0.0001).
Here we have an example of a clinical case.
Patient K., born 1957, was admitted to the gastroenterology department with the diagnosis “active hepatitis of alcoholic etiology”. According to the results of biochemical blood assay there was found the syndrome of cytolysis and cholestasis: total bilirubin — 22.5 µmol/L; ALAT — 75 U/L; AAT — 63 U/L; alkaline phosphatase — 119 U/L; GGT — 67 U/L.
Ultrasound investigation showed diffuse changes of parenchyma, the moderate increase of anteroposterior liver size (Fig. 1). Additional spleen ultrasound demonstrated the following: size — within normal range, echostructure — no changes, but v. lienalis dilatation (Fig. 2). Liver transient elastometry showed F3-stage fibrosis (Fig. 3), and additional compression elastography in endosonography enabled to diagnose liver cirrhosis (Fig. 4).
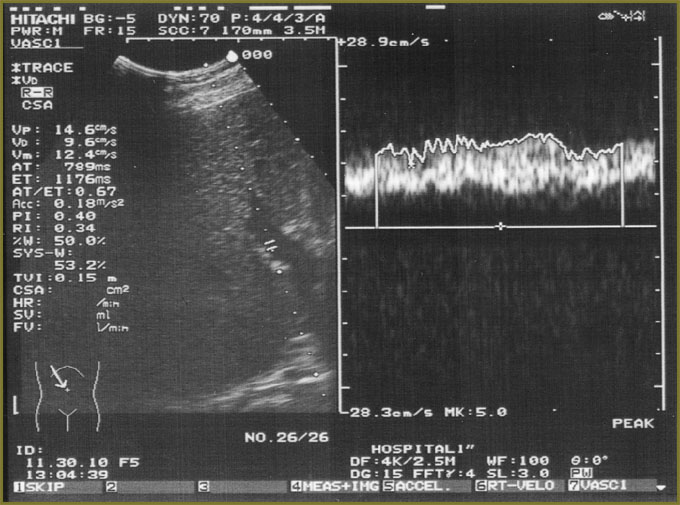 Fig. 1. Hepatic sonography: В-mode with portal hemodynamics assessment Fig. 1. Hepatic sonography: В-mode with portal hemodynamics assessment
|
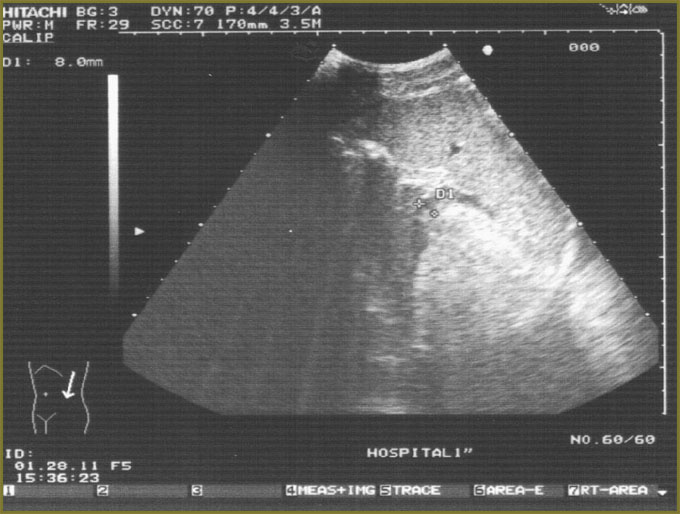 Fig. 2. Spleen sonography: В-mode with v. lienalis diameter assessment Fig. 2. Spleen sonography: В-mode with v. lienalis diameter assessment
|
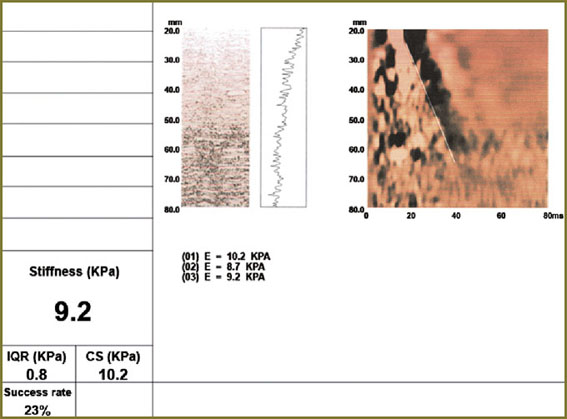 Fig. 3. Elastographic data (liver transient elastometry) Fig. 3. Elastographic data (liver transient elastometry)
|
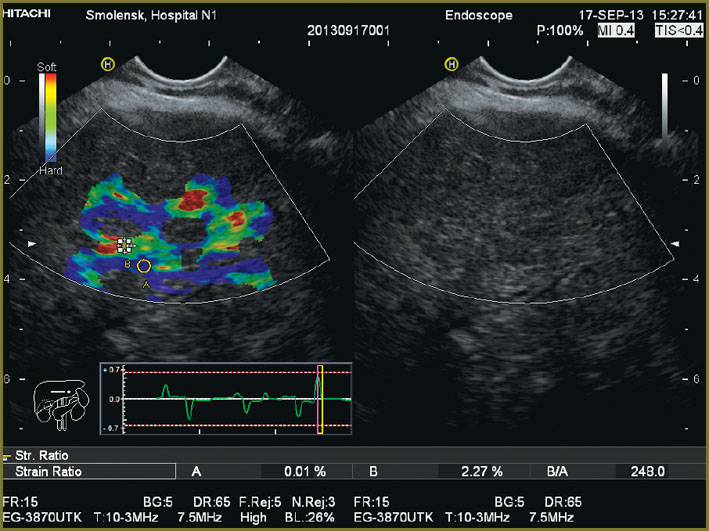 Fig. 4. Compression elastography in hepatic endosonography Fig. 4. Compression elastography in hepatic endosonography
|
The example shows that a gastroenterologist should be suspicious of a possible quick outcome of hepatitis in hepatic cirrhosis in such patients.
To confirm informativeness and diagnostic value of the investigations carried out we performed the discriminative analysis determining standardized canonical coefficient of discriminant function, which based on the above-mentioned methods enables to calculate their sensitivity, specificity and accuracy (Table 2).
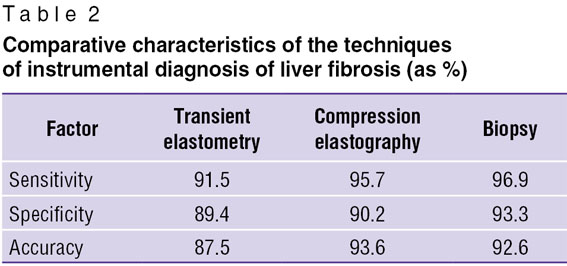 Table 2. Comparative characteristics of the techniques of instrumental diagnosis of liver fibrosis (as %) Table 2. Comparative characteristics of the techniques of instrumental diagnosis of liver fibrosis (as %)
|
The analysis showed higher values of sensitivity, specificity and accuracy of compression elastography in endosonography compared to transient elastometry. Virtually the same values of compression elastography in endosonography and liver biopsy indicate that biopsy is still a gold standard of liver diagnostics resulting in informative results in case it is technically feasible.
In the study there were determined optimal threshold values of compression elastography in steatosis, hepatitis, liver cirrhosis, when diagnostic sensitivity, specificity and accuracy are maximum. If difference coefficient is from 5 to 17 RU (F0-stage) and from 18 to 25 RU (F1-stage) the diagnosis of hepatic steatosis is confirmed, from 26 to 37 RU (F2-stage) and from 38 to 49 RU (F3-stage) — hepatitis, from 50 to 100 RU (F4-stage) — liver cirrhosis.
Thus, the study carried out demonstrated significant differential diagnostic possibilities and application prospects of compression elastography in endosonography, particularly in gastroenterology and hepatology.
Conclusion. Compression elastography in endosonography enables to image hepatic segments (segments 1, 2, 4, 5, 8), which are not always accessible in transabdominal sonography, and are inaccessible in transient elastometry; therefore, making it possible to improve early diagnostics of liver fibrosis process.
Study Funding and Conflict of Interests. The study was not supported by any financial sources, and the authors have no conflict of interest to disclose.
References
- Borsukov A.V., Kryukovskii S.B., Pokusaeva V.N., et al. Elastografiya v klinicheskoy gepatologii [Elastography in clinical hepatology]. Smolensk; 2011; 276 p.
- Borsukov A.V., Mamoshin A.V. Maloinvazivnye vmeshatel’stva pod ul’trazvukovym kontrolem pri zabolevaniyakh zhelchnogo puzyrya i podzheludochnoy zhelezy [Minimally invasive interventions under ultrasound control in gallbladder and pancreatic diseases]. Moscow: Publishing House “Medpraktika-M”; 2007; 128 p.
- Borsukov A.V., Alibegov R.A., Andreyev O.V., et al. Maloinvazivnyy elektrokhimicheskiy lizis v gepatologii, mammologii, urologii, endokrinologii [Minimally invasive electrochemical lysis in hepatology, mammology, urology, endocrinology]. Moscow: Publishing House “Medpraktika-M”; 2008; 316 p.
- Borsukov A.V., Dolgushin B.I., Kosyrev V.Y., et al. Maloinvazivnye tekhnologii pod ul’trazvukovoy navigatsiey v sovremennoy klinicheskoy praktike [Minimally invasive technology under ultrasound navigation in modern clinical practice]. Smolensk; 2009; 248 p.
- Vertkin A.L., Tikhonovskaya E.Yu., Skvortsova A.A., et al. Characteristics of clinical course and pharmacotherapy of alcoholic disease of liver, heart and brain in patients with somatic pathology. Lechashchiy vrach 2009; 2: 64–69.
- Glushenkov D.V., Galushko M.Y., Ivashkin V.T. Elastometry contribution to clinical assessment of liver cirrhosis severity. Rossiyskiy zhurnal gastroenterologii, gepatologii, koloproktologii 2008; 18(5): 84.
- Glushenkov D.V., Zolotarevskii V.B., Ivashkin V.T. Liver fibrosis assessment in patients with NASH using elastometry. Rossiyskiy zhurnal gastroenterologii, gepatologii, koloproktologii 2008; 18(1): 65.
- Glushenkov D.V., Konovalova O.N., Zolotarevskii V.B., Ivashkin V.T. Non-invasive diagnostics of liver fibrosis in the early stages of its development. Rossiyskiy zhurnal gastroenterologii, gepatologii, koloproktologii 2008; 18(5): 83.
- Glushenkov D.V., Maevskaya M.V., Ivashkin V.T. Elastometry and fibrotest capabilities in liver cirrhosis diagnosis (clinical surveillance). Rossiyskiy zhurnal gastroenterologii, gepatologii, koloproktologii 2008; 18(1, Suppl. 31): 9.
- Govorin N.V., Sakharov A.V. Methodological approaches to the study of alcohol mortality (regional aspect). Voprosy narkologii 2011; 2: 7–12.
- Golovanov Y.V., Petrakov A.V. Intrahepatic cholestasis diagnosis and treatment in chronic liver diseases. Terapevticheskiy arkhiv 2011; 2: 33–39.
- Ivashkin V.T. Complications of portal hypertension in liver cirrhosis. Rossiyskiy fiziologicheskiy zhurnal im. I.M. Sechenova 2009; 95(10): 1088.
- Ivashkin V.T., Lapina T.L. Gastroenterologiya: natsional’noe rukovodstvo [Gastroenterology: national guide]. Moscow: GEOTAR-Media; 2008; 524 p.
- Cohen J. Atlas endoskopii pishchevaritel’nogo trakta, vozmozhnosti vysokogo razresheniya i izobrazheniya v uzkom svetovom spektre [Atlas of digestive endoscopy, features of high resolution images in a narrow light spectrum]. Moscow: Logosfera; 2012; 360 p.
- Kubyshkin V.A., Karmazanovskij G.G., Grishankov S.A. Kistoznye opukholi podzheludochnoy zhelezy: diagnostika i lechenie [Cystic pancreatic tumors: diagnosis and treatment]. Moscow; 2013; 328 p.
- Lazebnik L.B., Vinnytsia E.V., Shaposhnikov N.K. Khomeriki S.G., Nikanorov A.V., Terekhin A.A., Vorob’eva N.N., Golovanova E.V. Diagnostic significance of ultrasonic elastometry in the evaluation of fibrosis in chronic diffuse liver diseases. Eksperimental’naya i klinicheskaya gastroenterologiya 2010; 5: 10–13.
- Lemeshko Z.A. Radiology in gastroenterology. Rossiyskiy zhurnal gastroenterologii, gepatologii, koloproktologii 2011; 1: 79–84.
- Nechipay A.M., Orlov S.Y., Fedorov E.D. EUSbuka: rukovodstvo po endoskopicheskoy ul’trasonografii [EUSbuka: endoscopic ultrasonography guide]. Moscow: Prakticheskaya meditsina; 2013; 400 p.
- Pavlov C.S., Konovalov D.V. Glushenkov O.N., Ivashkin V.T. Clinical application of noninvasive techniques of liver fibrosis assessment: the results of own researches in a multidisciplinary hospital. Klinicheskaya meditsina 2009; 11: 40–45.
- Sobin L.H., Gospodarovich M.К., Vittekind K. Klassifikatsiya zlokachestvennykh opukholey [Classification of malignant tumors]. Moscow: Logosfera; 2011; 304 p.
- Trufanova Y.M., Topilskaya N.V., Morozov S.V., Isakov V.A., Kaganov B.S. Ultrasonic features of liver and elastography in overweight patients. Eksperimental’naya i klinicheskaya gastroenterologiya 2010; 5: 19–26.
- Harness J.K., Visher D.B. Ul’trazvukovaya diagnostika v khirurgii. Osnovnye svedeniya i klinicheskoe primenenie [Ultrasound diagnostic in surgery. Basic information and clinical application]. Moscow; 2012; 597 p.
- Persico M., Capasso M., Persico E., et al. Suppressor of cytokine signaling 3 (SOCS3) expression and hepatitis С virus-related chronic hepatitis: Insulin resistance and response to antiviral therapy. Hepatology 2007; 46(4): 1009–1015, http://dx.doi.org/10.1002/hep.21782.
- Săftoiu A., Vilmann P., Ciurea T., et al. Dynamic analysis of EUS used for the differentiation of benign and malignant lymph nodes. Gastrointest Endosc 2007; 66(2): 291–300, http://dx.doi.org/10.1016/j.gie.2006.12.039.
- Salih H.R., Holdenrieder S., Steinle A. Soluble NKG2D ligands: prevalence, release, and functional impact. Front Biosci 2008; 13: 3448–3456, http://dx.doi.org/10.2741/2939.
- Samonakis D.N., Cholongitas E., Thalheimer U., et al. Hepatic venous pressure gradient to assess fibrosis and its progression after liver transplantation for HCV cirrhosis. Liver Transpl 2007; 13(9): 1305–1311, http://dx.doi.org/10.1002/lt.21227.
- Sand J., Valikovski A., Nordback I. Alcohol consumption in the country and hospitalization for acute alcohol pancreatitis and liver cirrhosis during a 20-year period. Alcohol Alcohol 2009; 44(3): 321–325, http://dx.doi.org/10.1093/alcalc/agn121.










