Quantitative Assessment of Hyaline Cartilage Elasticity During Optical Clearing Using Optical Coherence Elastography
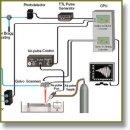
Tissue optical clearing is an emerging technique for dynamically modifying tissue optical properties to increase imaging depth, which is useful in applications such as imaging and functional diagnostics of many diseases. For example, optical clearing of cartilage allowed imaging of subchondral bone that is used to assess orthopedic diseases. However, the effect of the clearing processes on tissue elastic properties has not been investigated yet. In this study we report the first use of phase-stabilized swept source optical coherence elastography (PhS-SSOCE) to quantitatively monitor the change in elasticity of hyaline cartilage during the optical clearing process noninvasively. The results showed that PhS-SSOCE was able to assess the increase in cartilage stiffness during the clearing process over time and with different concentrations of glucose. In addition, the results demonstrated that the elasticity of the cartilage was reversed once the clearing agent was replaced with saline. To verify the results obtained from the PhS-SSOCE measurements, benchmark mechanical testing was performed using a uniaxial mechanical compression frame. Both methods demonstrated the same trend of the elasticity change of the cartilage immersed in glucose solution. The data show that during the transition from phosphate buffered saline to the clearing agent, the cartilage stiffness decreases significantly, which indicates that the clearing agent diffused into the cartilage extracellular matrix and decreased the tissue elasticity due to dehydration. Therefore, the proposed optical coherence elastography can dynamically assess the effects of optical clearing and associated changes in tissue biomechanical properties noninvasively and nondestructively. This technique may be potentially useful in orthopedic studies such as early detection and monitoring of osteoarthritic diseases.
- Zhu D., Larin K.V., Luo Q., Tuchin V.V. Recent progress in tissue optical clearing. Laser Photon Rev 2013; 7(5): 732–757, http://dx.doi.org/10.1002/lpor.201200056.
- Larin K.V., Ghosn M.G., Bashkatov A.N., Genina E.A., Trunina N.A., Tuchin V.V. Optical clearing for OCT image enhancement and in-depth monitoring of molecular diffusion. IEEE J Sel Top Quantum Electron 2012; 18: 1244–1259, http://dx.doi.org/10.1109/jstqe.2011.2181991.
- Zhao Q.L., Si J.L., Guo Z.Y., Wei H.J., Yang H.Q., Wu G.Y., Xie S.S., Li X.Y., Guo X., Zhong H.Q., Li L.Q. Quantifying glucose permeability and enhanced light penetration in ex vivo human normal and cancerous esophagus tissues with optical coherence tomography. Laser Phys Lett 2011; 8(1): 71–77, http://dx.doi.org/10.1002/lapl.201010081.
- Ghosn M.G., Sudheendran N., Wendt M., Glasser A., Tuchin V.V., Larin K.V. Monitoring of glucose permeability in monkey skin in vivo using optical coherence tomography. J Biophotonics 2010; 3(1–2): 25–33, http://dx.doi.org/10.1002/jbio.200910075.
- Larina I.V., Carbajal E.F., Tuchin V.V., Dickinson M.E., Larin K.V. Enhanced OCT imaging of embryonic tissue with optical clearing. USA, CA; 2009.
- Ghosn M.G., Carbajal E.F., Befrui N.A., Tuchin V.V., Larin K.V. Differential permeability rate and percent clearing of glucose in different regions in rabbit sclera. J Biomed Opt 2008; 13(2): 021110, http://dx.doi.org/10.1117/1.2907699.
- Ghosn M.G., Carbajal E.F., Befrui N.A., Tellez A., Granada J.F., Larin K.V. Permeability of hyperosmotic agent in normal and atherosclerotic vascular tissues. J Biomed Opt 2008; 13(1): 010505, http://dx.doi.org/10.1117/1.2870153.
- Larin K.V., Ghosn M.G., Ivers S.N., Tellez A., Granada J.F. Quantification of glucose diffusion in arterial tissues by using optical coherence tomography. Laser Phys Lett 2007; 4(4): 312–317, http://dx.doi.org/10.1002/lapl.200610111.
- Ghosn M.G., Tuchin V.V., Larin K.V. Nondestructive quantification of analyte diffusion in cornea and sclera using optical coherence tomography. Invest Ophthalmol Vis Sci 2007; 48(6): 2726–2733, http://dx.doi.org/10.1167/iovs.06-1331.
- Ghosn M.G., Tuchin V.V., Larin K.V. Depth-resolved monitoring of glucose diffusion in tissues by using optical coherence tomography. Opt Lett 2006; 31(15): 2314–2316, http://dx.doi.org/10.1364/OL.31.002314.
- Schmitt J.M. Optical coherence tomography (OCT): a review. IEEE J Sel Top Quantum Electron 1999; 5(4): 1205–1215, http://dx.doi.org/10.1109/2944.796348.
- Kennedy B.F., Kennedy K.M., Sampson D.D. A review of optical coherence elastography: fundamentals, techniques and prospects. IEEE J Sel Top Quantum Electron 2014; 20(2): 272–288, http://dx.doi.org/10.1109/jstqe.2013.2291445.
- Ko H.J., Tan W., Stack R., Boppart S.A. Optical coherence elastography of engineered and developing tissue. Tissue Eng 2006; 12(1): 63–73, http://dx.doi.org/10.1089/ten.2006.12.63.
- Wang S., Larin K.V. Shear wave imaging optical coherence tomography (SWI-OCT) for ocular tissue biomechanics. Opt Lett 2014; 39(1): 41–44, http://dx.doi.org/10.1364/OL.39.000041.
- Li J., Wang S., Singh M., Aglyamov S., Emelianov S., Twa M.D., Larin K.V. Air-pulse OCE for assessment of age-related changes in mouse cornea in vivo. Laser Phys Lett 2014; 11(6): 065601, http://dx.doi.org/10.1088/1612-2011/11/6/065601.
- Wang S., Li J., Manapuram R.K., Menodiado F.M., Ingram D.R., Twa M.D., Lazar A.J., Lev D.C., Pollock R.E., Larin K.V. Noncontact measurement of elasticity for the detection of soft-tissue tumors using phase-sensitive optical coherence tomography combined with a focused air-puff system. Opt Lett 2012; 37(24): 5184–5186, http://dx.doi.org/10.1364/OL.37.005184.
- Wang S., Lopez A.L., Morikawa Y., Tao G., Li J., Larina I.V., Martin J.F., Larin K.V. Noncontact quantitative biomechanical characterization of cardiac muscle using shear wave imaging optical coherence tomography. Biomed Opt Exp 2014; 5(7): 1980–1992, http://dx.doi.org/10.1364/boe.5.001980.
- Wang S., Liu C.-H., Zakharov V.P., Lazar A.J., Pollock R.E., Larin K.V. Three-dimensional computationalanalysis of optical coherence tomography images for the detection of soft tissue sarcomas. J Biomed Opt 2014; 19(2): 021102, http://dx.doi.org/10.1117/1.JBO.19.2.021102.
- Liu C.-H., Qi J., Lu J., Wang S., Wu C., Shih W.-C., Larin K.V. Improvement of tissue analysis and classification using optical coherence tomography combined with Raman spectroscopy. J Innov Opt Health Sci 2014; 1550006, http://dx.doi.org/10.1142/s1793545815500066.
- Wang S., Larin K.V., Li J., Vantipalli S., Manapuram R.K., Aglyamov S., Emelianov S., Twa M.D. A focused air-pulse system for optical-coherence-tomography-based measurements of tissue elasticity. Laser Phys Lett 2013; 10(7): 075605, http://dx.doi.org/10.1088/1612-2011/10/7/075605.
- Sobol E.N., Milner T.E., Shekhter A.B., Baum O.I., Guller A.E., Ignatieva N.Y., Omelchenko A.I., Zakharkina O.L. Laser reshaping and regeneration of cartilage. Laser Phys Lett 2007; 4(7): 488–502, http://dx.doi.org/10.1002/lapl.200710019.
- Diaz S.H., Aguilar G., Lavernia E.J., Wong B.J.F. Modeling the thermal response of porcine cartilage to laser irradiation. IEEE J Sel Top Quantum Electron 2001; 7(6): 944–951, http://dx.doi.org/10.1109/2944.983298.
- Ophir J., Alam S.K., Garra B.S., Kallel F., Konofagou E.E., Krouskop T., Merritt C.R.B., Righetti R., Souchon R., Srinivasan S., Varghese T. Elastography: imaging the elastic properties of soft tissues with ultrasound. J Med Ultrasonics 2002; 29(4): 155–171, http://dx.doi.org/10.1007/bf02480847.
- Peschke K.-D., Haasdonk B., Ronneberger O., Burkhardt H., Rösch P., Harz M., Popp J.R. Using transformation knowledge for the classification of Raman spectra of biological samples. In: Proceedings of the 24th IASTED international conference on biomedical engineering. 2006; p. 288–293.
- Ghosn M.G., Mashiatulla M., Mohamed M.A., Syed S., Castro-Chavez F., Morrisett J.D., Larin K.V. Time dependent changes in aortic tissue during cold storage in physiological solution. Biochim Biophys Acta 2011; 1810(5): 555–560, http://dx.doi.org/10.1016/j.bbagen.2011.02.003.
- Genina E.A., Bashkatov A.N., Tuchin V.V., Ghosn M.G., Larin K.V., Kamenskikh T.G. Cortexin diffusion in human eye sclera. Quantum Electron 2011; 41(5): 407–413, http://dx.doi.org/10.1070/qe2011v041n05abeh014641.
- Carbajal E.F., Baranov S.A., Manne V.G.R., Young E.D., Lazar A.J., Lev D.C., Pollock R.E., Larin K.V. Revealing retroperitoneal liposarcoma morphology using optical coherence tomography. J Biomed Opt 2011; 16(2): 020502, http://dx.doi.org/10.1117/1.3541789.
- Ivers S.N., Baranov S.A., Sherlock T., Kourentzi K., Ruchhoeft P., Willson R., Larin K.V. Depth-resolved imaging and detection of micro-retroreflectors within biological tissue using optical coherence tomography. Biomed Opt Exp 2010; 1(2): 367–377, http://dx.doi.org/10.1364/boe.1.000367.
- Manapuram R.K., Manne V.G.R., Larin K.V. Phase-sensitive swept source optical coherence tomography for imaging and quantifying of microbubbles in clear and scattering media. J Appl Phys 2009; 105(10): 102040, http://dx.doi.org/10.1063/1.3116614.
- Ghosn M.G., Leba M., Vijayananda A., Rezaee P., Morrisett J.D., Larin K.V. Effect of temperature on permeation of low-density lipoprotein particles through human carotid artery tissues. J Biophotonics 2009; 2(10): 573–580, http://dx.doi.org/10.1002/jbio.200810071.
- Ghosn M.G., Carbajal E.F., Befrui N.A., Tuchin V.V., Larin K.V. Concentration effect on the diffusion of glucose in ocular tissues. Optics and Lasers in Engineering 2008; 46(12): 911–914, http://dx.doi.org/10.1016/j.optlaseng.2008.05.004.
- Wong B.J., Chao K.K.H., Kim H.K., Chu E.A., Dao X., Gaon M., Sun C.-H., Nelson J.S. The porcine and lagomorph septal cartilages: models for tissue engineering and morphologic cartilage research. Am J Rhinol 2001, 15(2): 109–116, http://dx.doi.org/10.2500/105065801781543790.
- Chae Y., Aguilar G., Lavernia E.J., Wong B.J. Characterization of temperature dependent mechanical behavior of cartilage. Lasers Surg Med 2003; 32(4): 271–278, http://dx.doi.org/10.1002/lsm.10167.
- Jerabek M., Major Z., Lang R.W. Uniaxial compression testing of polymeric materials. Polymer Testing 2010; 29(3): 302–309, http://dx.doi.org/10.1016/j.polymertesting.2009.12.003.
- Padalkar M.V., Spencer R.G., Pleshko N. Near infrared spectroscopic evaluation of water in hyaline cartilage. Ann Biomed Eng 2013; 41(11): 2426–2436, http://dx.doi.org/10.1007/s10439-013-0844-0.
- Bagratashvili V.N., Sobol E.N., Sviridov A.P., Popov V.K., Omel’chenko A.I., Howdle S.M. Thermal and diffusion processes in laser-induced stress relaxation and reshaping of cartilage. J Biomech 1997; 30(8): 813–817, http://dx.doi.org/10.1016/S0021-9290(97)00028-6.
- Sobol E., Sviridov A., Omel’chenko A., Bagratashvili V., Kitai M., Harding S.E., Jones N., Jumel K., Mertig M., Pompe W., Ovchinnikov Y., Shekhter A., Svistushkin V. Laser reshaping of cartilage. Biotechnol Genet Eng Rev 2000; 17(1): 553–578, http://dx.doi.org/10.1080/02648725.2000.10648005.
- Liu C.H., Skryabina M.N., Li J., Singh M., Sobol E.N., Larin K.V. Measurement of the temperature dependence of Young’s modulus of cartilage by phase-sensitive optical coherence elastography. Quantum Electron 2014; 44(8): 751–156, http://dx.doi.org/10.1070/qe2014v044n08abeh015506.
- Oliveira L.M., Carvalho M.I., Nogueira E.M., Tuchin V.V. The characteristic time of glucose diffusion measured for muscle tissue at optical clearing. Laser Physics 2013; 23(7): 075606, http://dx.doi.org/10.1088/1054-660x/23/7/075606.
- Sobol E., Shekhter A., Guller A., Baum O., Baskov A. Laser-induced regeneration of cartilage. J Biomed Opt 2011; 16(8): 080902, http://dx.doi.org/10.1117/1.3614565.










