Neuromodulation for Refractory Failed Back Surgery Syndrome Patients
The aim of the investigation was to assess the capabilities of spinal cord stimulation, and compare it with conventional nonsurgical treatment in failed back syndrome.
Materials and Methods. The study involved the patients with at least one anatomically successful lumber disc hernia excision, post-traumatic spinal deformity in their past history, the patients keeping suffering from neuropathic pain.
Group 1 patients (n=45) in addition to traditional therapy underwent spinal cord stimulation. Group 2 control patients (n=35) were given medicines, therapeutic blocks, physiotherapy, and psychotherapy.
Results. Epidural spinal cord stimulation in chronic pain syndrome treatment enables to improve significantly the patients' condition: it lowers pain level, increases the functionality and improves the quality of life.
Conclusion. Being minimally invasive and highly safe, neuromodulation enables to achieve good treatment results in failed back syndrome.
The first spinal cord stimulation surgery in failed back syndrome was performed 47 years ago by Shealy to a patient with an end-stage cancer [1]. Stimulation means an electrode placed in the epidural space, the electrode being connected with an implanted neurostimulator initiating an electric field above the spinal structures. Multiple-contact electrodes to initiate an electric field are placed above the posterior columns of the spinal cord, and a programmable device makes it possible for a patient to control the parameter of a procedure using a remote control [2].
Since 1967 there has been performed over 200 thousand implantations of neurostimulators, now each year over 25 thousand stimulators are implanted due to a variety of indications [3]. In the last decade there has been the technology breakthrough in neuromodulation application.
Every year axial pain syndrome is responsible for spinal surgeries in more than a million of patients. Unfortunately, 40% surgeries fail, and patients are still suffering from postoperative chronic pain [4]. Some of these patients are diagnosed “failed back surgery syndrome” characterized by scelalgia, gluteal or low back pain [5]. Spinal pathology correction can be a success, while a long-term analgetic effect is difficult to achieve that can be adequately explained by academician Kryzhanovskiy theory of pathological pain system [6]. Patients suffering from chronic pain after multiple spinal surgeries have distress, sleep disorders, impaired functional capabilities, and other accompanying disorders frequently resulting in job loss, decreased social activity and social skills. Traditionally, the conservative treatment is applied in failed back surgery syndrome: drug therapy including therapeutic blocks and epidural steroid injections, physiotherapeutic procedures. However, most patients are irresponsive to these therapies, and due to this fact, neurostimulation is indicated in failed back syndrome [5, 7].
The aim of the investigation was to assess the capabilities of spinal cord stimulation, and compare it with conventional nonsurgical treatment in failed back syndrome.
Materials and Methods. The study involved the patients with at least one anatomically successful lumbar disc hernia excision, lumbar stenosis correction, spondylolisthesis, post-traumatic spinal deformity in their past history, they keeping suffering from neuropathic pain more felt in legs rather than in the back, in dermatome L4, L5, S1 area, with no negative dynamics of neurologic impairment. The inclusion criterion was the presence of the pain syndrome, its intensity being at least 50 mm according to Visual analogue scale, where 0 means no pain, and 100 mm implies the most intense pain [8].
The exclusion criteria were non-corrected compression of nervous structures, nociceptive pain, psychiatric or behavior disorders, untapped resources of medicinal treatment.
The study involved 80 patients aged 16–76 years (mean age 44 years), the period of suffering being 2–30 years (mean age 7.5 years). The patients were randomized into two groups comparable in sex, age and clinical presentation intensity. Group 1 patients (n=45) in addition to traditional therapy underwent spinal cord stimulation. Group 2 control patients (n=35) were given medicines (non-steroid anti-inflammatory drugs, antidepressants, antiepileptic agents), therapeutic blocks, physiotherapy and psychotherapy. Group 1 patients were implanted an epidural electrode and a pulsed generator Medtronic (USA) to stimulate spinal cord if during a test period at least 80% pain area was overlapped by paresthesiae, and there was at least double decreased pain intensity. The patients were operated at Neurosurgery Department of Privolzhsky Federal Research Medical Centre, Ministry of Health of the Russian Federation in 2012–2014.
The study complies with the declaration of Helsinki (adopted in June, 1964 (Helsinki, Finland) and revised in October, 2000 (Edinburg, Scotland)) and was approved by the Ethics Committee of Privolzhsky Federal Research Medical Centre, Ministry of Health of the Russian Federation. Written informed consent was obtained from all patients.
Epidural electric spinal stimulation included percutaneous implantation of 4- or 8-contact test electrode under local anesthesia and X-ray control (Figure 1), it being placed along a physiological median line of the spine (Figure 2) followed by activation of positive and negative contacts by a successive displacement of electric dipoles along the electrode. By increasing the stimulation amplitude we succeeded in achieving comfortable paresthesia covering the pain area indicated by a patient. If we failed to achieve sensations appropriate in localization and a pain relieving effect, the electrode position was changed. The upper contact of the electrode situated at ThIX–X was considered optimal. 50% pain decrease compared to that during the test simulation (3–10 days) was taken as a sufficient criterion to implant a permanent electrode and a pulsed generator.
 Figure 1. C-arm-guided percutaneous electrode implantation Figure 1. C-arm-guided percutaneous electrode implantation
|
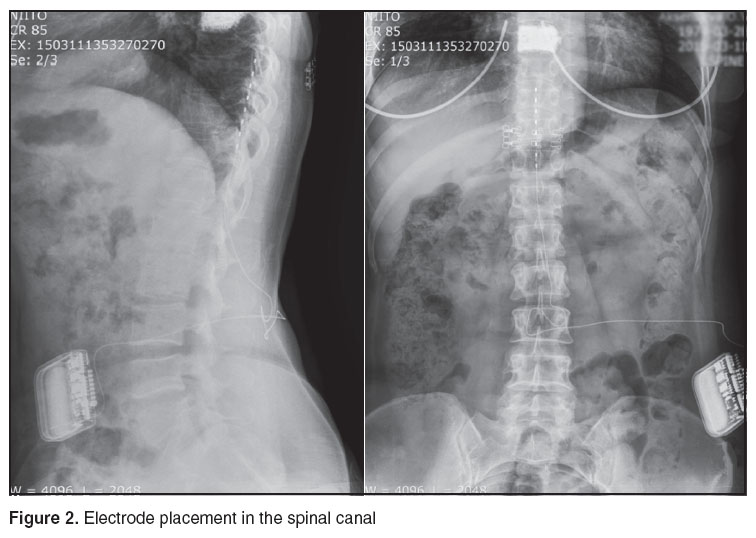 Figure 2. Electrode placement in the spinal canal Figure 2. Electrode placement in the spinal canal
|
The procedure was considered effective if 6 month after the surgery the pain intensity in the leg remained 50% decreased compared to initial. The patients assessed the pain intensity according to Visual analogue scale. For life activity failure assessment due to pain syndrome we used Oswestry questionnaire [8].
The findings were statistically processed using Statistica 10.0. Provided that the distribution of parameters was no different from normal we applied parametrical methods: a median and an inter-quartile range (Ме [25%; 75%]) were calculated. The hypothesis for quantitative changes was tested by Wilcoxon criterion. The critical significance point in testing statistical hypotheses was equal to 0.05.
Results. Five of 45 patients in group 1 had no effect of the test stimulation, thus only 40 patients underwent the implantation of a permanent electrode and a pulsed generator. The results were assessed 2 weeks and 6 months after the electrode implantation.
Two weeks after the procedure 27 patients in group 1 (67.5%) and 5 control patients (14.3%) showed at least 50% pain syndrome regress (p<0.001).
Six months after neurostimulation application the pain intensity in leg and back in group 1 patients was lower, and treatment satisfaction was higher compared to the control group (p=0.001). In particular, according to Visual analogue scale, pain regress changed from 7.9 [10.0; 5.0] to 5.1 [6.0; 2.0] in group 1, and from 8.0 [10.0; 5.0] to 6.9 [10.0; 5.0] in the control group. 32 patients (80%) in group 1 were satisfied with a pain decease ratio, while among control patients there were only 7 satisfied patients (20%). Moreover, 4 patients in group 1 discontinued the intake of semi-narcotic analgesics (tramadol) and 6 patients discontinued anticonvulsants (gabapentin). Daily living quality against the decreased pain syndrome (according to Oswestry questionnaire) six months after the procedure was significantly higher (p=0.017) in group 1 patients: the score under 10 points of the questionnaire decreased from 31 [14; 40] to 17 [8; 26]. The control patients showed less significant recovery of daily living: from 32 [16; 39] to 26 [14; 38] scores. Thus, the findings count in favor of spinal cord stimulation in patients with failed back surgery syndrome: 80% of such patients and only 20% patients with conventional treatment achieve 50% decrease of pain intensity.
During the follow-up there were observed 8 complications related to the appliances used. Infected wound/abscess occurred in 4 patients, and 4 patients required reoperation, repeated electrode replacement (Figure 3), due to the electrode migration. It should be noted that these complications are considered to be the most common in neurostimulator implantation [9].
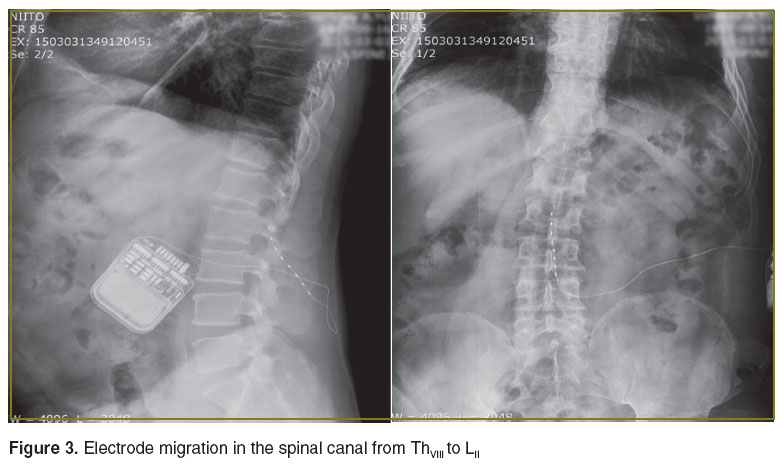 Figure 3. Electrode migration in the spinal canal from ThVIII to LII Figure 3. Electrode migration in the spinal canal from ThVIII to LII
|
Conclusion. Spinal cord stimulation in patients with neuropathic pain in failed back surgery syndrome compared to traditional methods significantly decreases the pain intensity, improves functional capabilities and activity in daily living, and increases the treatment satisfaction.
Study Funding. The study was not funded by any sources.
Conflicts of Interest. The authors have no conflicts of interest related to the present study.
References
- Shealy C.N., Mortimer J.T., Reswick J.B. Electrical inhibition of pain by stimulation of the dorsal columns: preliminary clinical report. Anesth Analg 1967; 46(4): 489–491, http://dx.doi.org/10.1213/00000539-196707000-00025.
- Molnar G., Barolat G. Principles of cord activation during spinal cord stimulation. Neuromodulation 2014; 17(Suppl 1): 12–21, http://dx.doi.org/10.1111/ner.12171.
- Kreis P., Fishman S. Spinal cord stimulation percutaneous implantation techniques. New York: Oxford University Press; 2009.
- Morozov I.N. Vosstanovitel'noe lechenie bol'nykh posle operativnogo udaleniya gryzh mezhpozvonkovykh diskov poyasnichnogo otdela pozvonochnika. Avtoref. dis. … kand. med. nauk [Medical rehabilitation of patients after lumber disc hernia excision. Absract of PhD thesis]. Nizhny Novgorod; 2001.
- Schu S., Slotty P.J., Bara G., von Knop M., Edgar D., Vesper J. A prospective, randomized, double-blind, placebo-controlled study to examine the effectiveness of burst spinal cord stimulation patterns for the treatment of failed back surgery syndrome. Neuromodulation 2014; 17(5): 443–450, http://dx.doi.org/10.1111/ner.12197.
- Kryzhanovskiy G.N. Determinantnye struktury v patologii nervnoy sistemy: generatornye mekhanizmy neyropatologicheskikh sindromov [Determinant structures in nervous system pathology: generator mechanisms of neuropathological syndromes]. Moscow: Meditsina; 1980; 360 p.
- Zucco F., Lavano A. Spinal cord stimulation for refractory failed back surgery syndrome patients: a cost-effective treatment in Italy (precise study). In: INS 11th World Congress in Berlin. Neuromodulation: technology transforming chronic illness management. Berlin; 2013.
- Belova A.N. Shkaly, testy i oprosniki v nevrologii i neyrokhirurgii [Scales, tests and questionnaires in neurology and neurosurgery]. Moscow; 2014; 434 p.
- Deer T.R., Mekhail N., Provenzano D., Pope J., Krames E., Thomson S., Raso L., Burton A., DeAndres J., Buchser E., Buvanendran A., Liem L., Kumar K., Rizvi S., Feler C., Abejon D., Anderson J., Eldabe S., Kim P., Leong M., Hayek S., McDowell G. 2nd, Poree L., Brooks E.S., McJunkin T., Lynch P., Kapural L., Foreman R.D., Caraway D., Alo K., Narouze S., Levy R.M., North R.; Neuromodulation Appropriateness Consensus Committee. The appropriate use of neurostimulation: avoidance and treatment of complications of neurostimulation therapies for the treatment of chronic pain. Neuromodulation 2014; 17(6): 571–597; discussion 597–598, http://dx.doi.org/10.1111/ner.12206.










