In vitro Cytokine Synthesis by Lymphocytes in Children in Juvenile Idiopathic Arthritis Remission Against the Background of Genetically Engineered Biologic Drug Therapy
The aim of the investigation to assess the capacity of lymphocytes for spontaneous and stimulated cytokine production in vitro in children in remission with juvenile rheumatoid arthritis treated with genetically engineered biological agents combined with immunosuppressants.
Materials and Methods. We examined 18 children (8 girls and 10 boys) aging from 2 to 12 years with various types of juvenile idiopathic arthritis treated with genetically engineered biological agents combined with immunosuppressants, no glucocorticoids were administered. The mean duration of genetically engineered biological agent therapy was 3.8 years. When included in the study all patients were recorded to have the disease remission according to Wallace criteria. Twelve age-matched children without chronic diseases and medical therapy were included in the control group. We assessed spontaneous and phytohemagglutinin (PHA)-induced cytokine synthesis (IL-2, IL-4, IL-6, IL-8, IL-10, G-CSF, IFN-γ, TNF-α), as well as their synthesis stimulation index. Cytokine concentration was determined in whole blood cultures using multiplex test systems (Bio-Plex Pro Human Cytokine) for Bio-Plex 200 (Bio-Plex Manager, version 5.0) (Bio-Rad, USA).
Results. We compared the activity of spontaneous and stimulated cytokine synthesis in children with juvenile idiopathic arthritis and controls to find a significant decrease in the spontaneous synthesis of IL-4, IL-6, IL-8, G-CSF, IFN-γ, TNF-α, and no significant differences in spontaneous production of IL-2 and IL-10. The analysis of PHA-stimulated cultures in children with arthritis showed the higher production of IL-2, IL-4, IL-6, the decreased IL-10 production, and no significant differences in the production of IL-8, G-CSF, IFN-γ, TNF-α. The calculated stimulation indices of G-CSF, IL-8, and TNF-α were similar in both groups of children, while those of IL-2, IL-4, IL-6, IFN-γ appeared to be significantly higher, and the indices for IL-10 were reduced.
Conclusion. No significant differences in stimulated synthesis levels of IL-8, G-CSF, IFN-γ, TNF-α in patients with juvenile idiopathic arthritis and in controls, and the increased stimulated IL-2, IL-4, IL-6 synthesis in children with arthritis suggest the preserved reactivity during a long-term therapy with genetically engineered biological agents in children with juvenile idiopathic arthritis.
The question to what extent functional capabilities of the immune system are inhibited in long-term immunosuppressive therapy is an important problem when developing an optimal management of patients with many autoimmune diseases, in particular those with juvenile idiopathic arthritis (JIA). JIA is a multi-factorial disease with generalized involvement of locomotor apparatus, progressive osteochondral destruction of joints, and incompetence developed. The most severe systemic form of the disease is accompanied by the immunopathological involvement of internal organs, when carditis, serositis, interstitial lung disease can develop. Many cell populations participate in pathological changes in JIA including В-cells, Т-cells, dendritic cells, macrophages, monocytes and fibroblasts [1, 2]. Thus, the disease results from impaired multilevel cell-cell interaction. Currently, modern medications have been developed and widely used to treat JIA: genetically engineered biological agents (GEBA): TNF-α inhibitors, anti-В-cell agents, IL-6 receptor antagonists, which target and inhibit certain stage of a pathological immune response. The studies carried out have proved their high efficiency in rheumatoid arthritis in adult patients and children with JIA. The wide use of GEBA has significantly improved JIA prognosis and clinical course [3].
Infectious complications are high on the list of side effects of many conventional immunosuppressive therapy schemes previously used to treat systemic JIA with a long-term hormone therapy, methothrexate or methothrexate combined with cyclosporin being the basic therapy [4–8]. GEBA therapy is not only more effective but it is also characterized by lower frequency of severe unwanted sequelae [9, 10]. Considering the necessity for long-term (within several years) GEBA therapy, it is of current interest to study the immune system capability to maintain adequate anti-infective protection under long-term immunosuppression.
All the above mentioned determines the importance of estimating the functional state of different components of immune system in children with JIA under long-term immunosuppressive therapy using modern biological agents.
The aim of the investigation to assess the capacity of lymphocytes for spontaneous and stimulated cytokine production in vitro in children in remission with juvenile rheumatoid arthritis treated with genetically engineered biological agents combined with immunosuppressants.
Materials and Methods. The study involved 18 children (8 girls and 10 boys) aging from 2 to 12 years with various JIA types, the patients receiving immunosuppressive therapy according to protocols used in children rheumatologic practice [1, 11]. The study complies with the declaration of Helsinki (adopted in June, 1964 (Helsinki, Finland) and revised in October, 2000 (Edinburg, Scotland)) and was approved by the Ethics Committee of Scientific Children Health Centre of the Ministry of Health of the Russian Federation. Parents of all patients gave their written informed consent.
All children were treated by various GIBA: TNF-α inhibitors (infliximab, adalimumab, etanercept), CD20 receptor antibodies (rituximab), and IL-6 receptor antagonists (tocilizumab) combined with methothrexate. Average duration of biological agent therapy was 3.8 years. At the moment when the patients were included into the study, they were in a state of remission according to Wallace criteria (2011) [12]. During the study all the patients showed no signs of secondary immunodeficiency, the signs being the following: frequent long-standing, reluctant to adequate causal treatment infectious inflammatory diseases including those caused by opportunistic microorganisms, as well as poor wound healing, chronic diarrhea and malabsorption [13].
A control group included 12 children without chronic diseases, who received no drug therapy and were examined when undergoing vaccinal prevention. Both groups were comparable in age and sex.
The study was designed as retrospective, observational, cohort, with a control group.
Research methods. We studied the whole blood culture of JIA patients and controls according to a standard technique used for Quantiferon test (the study was designed for clinical indications). 1 ml blood was drawn in each patient by standard venipuncture technique in two special tubes. A test tube (positive control) contained phytohemagglutinin (PHA) sorbed on walls; a tube for negative control had no simulators. The tubes were incubated at 37°С for 24 h followed by 15-minute centrifugation at 2,000 g. After the procedure, plasma (100 µl) was taken and kept at –70°С for further cytokine level determination.
Cytokine concentration was determined by flow fluorimetry using fluorescent labeled microparticles as a solid phase, on a double-beam laser analyzer Bio-Plex 200 (Bio-Rad, USA) with Bio-Plex Manager, version 5.0, using ready multiplex test-systems of the same manufacturer (Bio-Plex Pro Human Cytokine).
The following parameters were assessed:
spontaneous and PHA-induced production of cytokines IL-2, IL-4, IL-6, IL-8, IL-10, G-CSF, IFN-γ, TNF-α;
stimulation index (SI): the ratio of cytokine production level after PHA stimulation to the level of their production in unstimulated cell culture.
Data processing. The results were analyzed using Statistica 6.0. (StatSoft Inc., USA). The findings here are presented in the form of median and 25 and 75% quartiles (Q). Mann–Whitney test was used to determine the significant differences of independent variables. The differences were considered significant if p≤0.05. Pearson correlation coefficient (r) estimated the direction and binding force of variables. The findings were considered significant if p≤0.05.
Results. Among the patients with JIA there were children with neither leukopenia nor lymphopenia. Absolute count of leukocytes at the moment of examination in all patients was within the age norm, accounting (6–15)·109/L. Absolute count of lymphocytes was (1.5–3.6)·109/L, and just two children were found to have their moderate decrease: (1.0–1.4)·109/L.
IL-2 spontaneous level had no significant differences in JIA patients and controls (See the Table); IL-2 production after simulation was significantly higher in children with arthritis than that in controls: 132.2 (104.8; 266.8) pg/ml. SI for IL-2 appeared to be significantly higher in children with JIA.
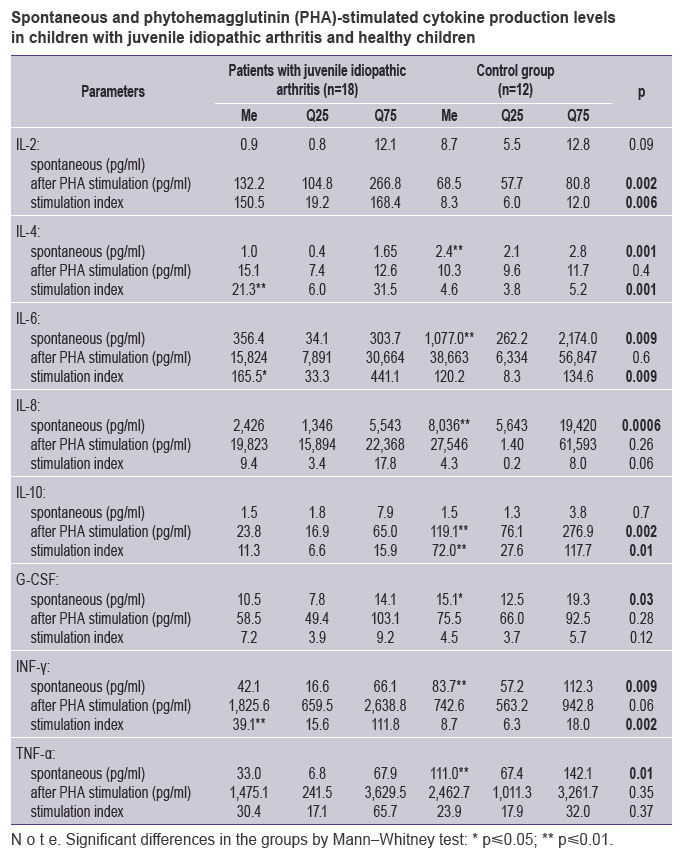 Spontaneous and phytohemagglutinin (PHA)-stimulated cytokine production levels in children with juvenile idiopathic arthritis and healthy children Spontaneous and phytohemagglutinin (PHA)-stimulated cytokine production levels in children with juvenile idiopathic arthritis and healthy children
|
IL-4 spontaneous synthesis level in JIA patients was relatively low accounting 1.0 (0.4; 1.65) pg/ml, while in the control group it was 2.5 (2.1; 2.8) pg/ml (p=0.0006). IL-4 stimulated production in both groups did not differ significantly, due to which SI for IL-4 in patients exceeded significantly compared to that in the controls: 21.3 (6.0; 31.5) and 4.6 (3.8; 5.2), respectively (p=0.006) (See the Table).
Spontaneous synthesis of IL-6 and IL-8 in children with JIA was also lower than that in the control group. The parameters of stimulated synthesis showed no significant differences. SI for IL-6 and IL-8 in both groups had no significant differences either (See the Table).
IL-10 spontaneous synthesis level in JIA patients and in the controls had no significant differences, while the stimulated production level and SI appeared to be significantly lower compared to those in the control group.
Spontaneous production of G-CSF and IFN-γ in patients with JIA was lower than that in the controls. G-CSF stimulated synthesis and its SI had no significant differences in both groups. IFN-γ stimulated synthesis showed a clear tendency for increase in JIA patients, SI median of this cytokine turned out to be significantly higher than in the control group (39.1 versus 8.7 pg/ml; p=0.002).
The median of TNF-α spontaneous production in patients with JIA was lower than that in the control group (33.0 versus 111.0 pg/ml; p=0.01), however, neither significant differences of stimulated production nor SI of the cytokine were found.
The dependence of cytokine production on lymphocyte count in a sample was assessed using the correlation analysis, which showed the positive correlation between the absolute count of lymphocytes in a sample and stimulated IL-4 production (r=0.5; p=0.02) and IL-6 (r=0.5; p=0.04), as well as SI of IL-2 (r=0.6; p=0.003), IL-4 (r=0.5; p=0.04), IL-6 (r=0.7; p=0.003), IL-10 (r=0.5; p=0.04), G-CSF (r=0.7; p=0.001), INF-γ (r=0.5; p=0.02), TNF-α (r=0.6; p=0.004). No significant correlations between the levels of produced cytokines and monocyte levels were found.
Discussion. Pathogenetic mechanisms of systemic JIA affect many components of immune system, and are associated with impaired tolerance to their own antibodies resulting in the activation of T and B lymphocytes, as well as macrophages. The activation leads to the altered production of many cytokines, and in its turn, enhances pathological cell reactions [1, 2].
18 of the examined children had different JIA forms, GEBA therapy being provided for a long period of time. The analysis of cytokine concentration in whole blood culture of these patients showed generally preserved ability of immune competent cells to respond adequately to nonspecific PHA stimulation. It was clearly demonstrated by high PHA-induced production of IL-2, IL-4, IL-6.
The comparison of spontaneous and stimulated cytokine production activity in children with a long-term JIA therapy according to modern protocols using GEBA [1, 11], with similar parameters in children with no chronic diseases reveled the significant decrease of IL-4, IL-6, IL-8, G-CSF, IFN-γ, TNF-α spontaneous synthesis, and no significant differences of IL-2, IL-10 spontaneous production. PHA-stimulated cultures in children with JIA were found to have higher production of IL-2, IL-4, IL-6, reduced production of IL-10, and no significant differences in IL-8, G-CSF, IFN-γ, TNF-α production. As a result, SI for G-CSF and TNF-α in JIA children did not differ from that in the controls, and SI was significantly higher for IL-2, IL-4, IL-6, IFN-γ, and decreased SI for L-10. No significant differences in stimulated production of IL-8, G-CSF, IFN-γ, TNF-α in JIA patients and controls in combination with increased stimulated IL-2, IL-4, IL-6 synthesis suggest the preserved responsiveness under a long-term immunosuppressive therapy without glucocorticosteroids.
Since we studied the whole blood culture, monocytes along with lymphocytes participated in the production of cytokines. However, there were found no significant correlations between the cytokine production level and the level of monocytes in blood. It suggests the contribution of monocytes in the synthesis activation of the cytokines under study using the described technique to be minimal.
When analyzing whole blood it is entirely possible that there is the excursion of cytokine synthesis level depending on lymphocyte content in an individual sample, and low total production can be related to drug-induced lymphopenia. In our study, at the moment of examination, the leukocyte count in all patients corresponded to the age norms: (6–15)·109/L; absolute count of lymphocytes in all patients was within the normal range (1.5–3.6)·109/L, only two children were found to have moderately decreased values: (1.0–1.5)·109/L. Supposing that the main reason for moderate decrease of spontaneous production of IL-4, IL-6, IL-8, G-CSF, IFN-γ, TNF-α can be relatively low absolute count of lymphocytes in blood samples studied, then stimulated production, which is no different from that in the control group, and high stimulation indices give evidence of both: preserved capacity, and, probably, the enhanced capability of the lymphocytes of the examined patients to respond to antigenic stimulation. It can be considered in two ways: both as a sign of no drug inhibition of lymphocyte function, and also the capability to activate immune responses (including the exacerbation of the underlying disease) under significant antigenic stimulation.
The data on preserved proliferative lymphocyte responsiveness to mitogen influence suggest the possibility of vaccinal prevention in JIA patients, if they have clinical laboratory remission. The studies [14, 15] have shown that vaccination of patients with rheumatoid arthritis against influenza, diphtheria, tetanus, and meningococcal infection has not resulted in the clinical setting deterioration. However, simultaneous use of supporting immunosuppressive therapy at average age doses was found not to inhibit the production of protective antibodies when vaccinated against diphtheria [15, 16], whooping cough [15, 17], hepatitis В [15, 18], influenza [15, 19]. When vaccinated against meningococcal infection, children with JIA also demonstrated the ability to synthesize protective antibodies, though their immune response was less intense than that in healthy children. Moreover, the patients with anti-TNF-α therapy combined with methothrexate appeared to have marked suppression of immune response compared to the cases with methothrexate administration alone [15, 20].
Thus, the obtained in the study preserved cell-mediated response to mitogenic stimulation in JIA patients holds out a hope that if the patients are vaccinated during clinical laboratory remission when under a long-term GEBA therapy combined with methothrexate, there can be an adequate vaccinal response.
Conclusion. The study results of in vitro stimulated cytokine synthesis by lymphocytes in children in juvenile idiopathic arthritis remission suggest preserved capability of immune system to respond to antigenic stimulation during a long-term therapy with genetically engineered biological agents. It is an additional advantage of this therapy, and in our view, can be taken into account when deciding upon the management of children with juvenile idiopathic arthritis.
Study Funding and Conflicts of Interest. The study was not funded by any sources, and the authors have no conflicts of interest related to the present study.
References
- Alekseeva E.I., Litvitskiy P.F., Baranov A.A. Yuvenil’nyy revmatoidnyy artrit: etiologiya, patogenez, klinika, algoritmy diagnostiki i lecheniya [Juvenile rheumatoid arthritis: etiology, pathogenesis, clinical features, diagnosis and treatment algorithms]. Pod red. Baranova A.A. [Baranov A.A. (editor)]. Moscow; 2007.
- Alekseeva E.I., Valieva S.I., Akulova S.S., Bzarova T.M., Denisova R.V., Isaeva K.B., Sleptsova T.V., Mitenko E.V., Chistyakova E.G., Fetisova A.N., Semikina E.L. Efficacy and safety of prolonged rituximab treatment in patients with systemic juvenile idiopathic arthritis. Voprosy sovremennoy pediatrii 2013; 12(2): 89–100.
- Yokota S., Imagawa T., Miyamae T. Safety and efficacy of up to three years of continuous tocilizumab therapy in children with systemic-onset juvenile idiopathic arthritis [SAT0536]. Ann Rheum Dis 2009; 68(Suppl. 3): 715.
- Smitten A.L., Choi H.K., Hochberg M.C., Suissa S., Simon T.A., Testa M.A., Chan K.A. The risk of hospitalized infection in patients with rheumatoid arthritis. J Rheumatol 2008; 35(3): 387–393.
- Doran M.F., Crowson C.S., Pond G.R., O’Fallon W.M., Gabriel S.E. Frequency of infection in patients with rheumatoid arthritis compared with controls: a population-based study. Arthritis Rheum 2002; 46(9): 2287–2293, http://dx.doi.org/10.1002/art.10524.
- Au K., Reed G., Curtis J.R., Kremer J.M., Greenberg J.D., Strand V., Furst D.E.; CORRONA Investigators. High disease activity is associated with an increased risk of infection in patients with rheumatoid arthritis. Ann Rheum Dis 2011; 70(5): 785–791, http://dx.doi.org/10.1136/ard.2010.128637.
- Doran M.F., Crowson C.S., Pond G.R., O’Fallon W.M., Gabriel S.E. Predictors of infection in rheumatoid arthritis. Arthritis Rheum 2002; 46(9): 2294–2300, http://dx.doi.org/10.1002/art.10529.
- Beukelman T., Xie F., Chen L., Baddley J.W., Delzell E., Grijalva C.G., Lewis J.D., Ouellet-Hellstrom R., Patkar N.M., Saag K.G., Winthrop K.L., Curtis J.R.; SABER Collaboration. Rates of hospitalized bacterial infection associated with juvenile idiopathic arthritis and its treatment. Arthritis Rheum 2012; 64(8): 2773–2780, http://dx.doi.org/10.1002/art.34458.
- Bzarova T.M., Alekseyeva Ye.I., Valiyeva S.I., Alekseyeva A.M., Denisova R.V., Isayeva K.B., Chistyakova Ye.G., Lisitsyn A.O. Safety of tumor necrosis factor blockers in adult’s and children’s rheumatologic practice. Voprosy sovremennoy pediatrii 2010; 9(1): 82–94.
- Halbig M., Horneff G. Improvement of functional ability in children with juvenile idiopathic arthritis by treatment with etanercept. Rheumatol Int 2009; 30(2): 229–238, http://dx.doi.org/10.1007/s00296-009-0942-3.
- Alekseyeva Ye.I., Bzarova T.M. Algorithm of diagnostics and treatment of juvenile arthritis. Voprosy sovremennoy pediatrii 2010; 9(6): 78–104.
- Wallace C.A., Giannini E.H., Huang B., Itert L., Ruperto N.; Childhood Arthritis Rheumatology Research Alliance; Pediatric Rheumatology Collaborative Study Group; Paediatric Rheumatology International Trials Organisation. American College of Rheumatology provisional criteria for defining clinical inactive disease in select categories of juvenile idiopathic arthritis. Arthritis Care Res 2011; 63(7): 929–936, http://dx.doi.org/10.1002/acr.20497.
- Khaitov R.M., Yarilin A.A., Pinegin B.V. Immunologiya: atlas [Immunology: an atlas]. Moscow; 2011.
- Pileggi G.S., de Souza C.B., Ferriani V.P. Safety and immunogenicity of varicella vaccine in patients with juvenile rheumatic diseases receiving methotrexate and corticosteroids. Arthritis Care Res 2010; 62(7): 1034–1039, http://dx.doi.org/10.1002/acr.20183.
- Dell’ Era L., Esposito S., Corona F., Principi N. Vaccination of children and adolescents with rheumatic diseases. Rheumatology 2011; 50(8): 1358–1365, http://dx.doi.org/10.1093/rheumatology/ker102.
- Koshcheeva Iu.V., Kharit S.M., Kalinina N.M. Specific features of diphtheria vaccinal process in children with rheumatic diseases. Zh Mikrobiol Epidemiol Immunobiol 2001; 6: 44–49.
- Kostinov M.P., Tarasova A.A., Zaitsev E.M. Contents of antibodies to Bordetella pertussis antigens in patients with rheumatic diseases. Zh Mikrobiol Epidemiol Immunobiol 2007; 6: 61–64.
- Kasapçopur O., Cullu F., Kamburoğlu-Goksel A., Cam H., Akdenizli E., Calýkan S., Sever L., Arýsoy N. Hepatitis B vaccination in children with juvenile idiopathic arthritis. Ann Rheum Dis 2004; 63(9): 1128–1130.
- Kanakoudi-Tsakalidou F., Trachana M., Pratsidou-Gertsi P., Tsitsami E., Kyriazopoulou-Dalaina V. Influenza vaccination in children with chronic rheumatic diseases and long-term immunosuppressive therapy. Clin Exp Rheumatol 2001; 19(5): 589–594.
- Elkayam O., Caspi D., Reitblatt T., Charboneau D., Rubins J.B. The effect of tumor necrosis factor blockade on the response to pneumococcal vaccination in patients with rheumatoid arthritis and ankylosing spondylitis. Semin Arthritis Rheum 2004; 33(4): 283–288, http://dx.doi.org/10.1053/j.semarthrit.2003.10.003.










