Transitory Increase of Hematoencephalic Barrier Permeability by Intracarotid Introduction of Ozonized Saline Solution
The aim of the investigation was to assess experimentally the effect of ozonized saline solution with various concentrations of ozone on the dynamics of hematoencephalic barrier (HEB) permeability.
Materials and Methods. Investigation of the ultrastructure of the sensorimotor zone of animal cerebral cortex to assess HEB state was carried out on mature outbred Wistar rats (n=93). In the control series, 0.9% saline solution was introduced intracarotidly, in the experimental series ozonized saline solution (OSS) at 0.7 and 3.5 mg/L ozone concentration was injected. The tissue of the rat sensorimotor cerebral cortex was examined morphologically 15 min, 1, 14 and 30 days after the solution introduction. The analysis of HEB permeability was evaluated by the state of astroglia, basement membrane, and intercellular junctions of capillary endothelium.
Results. It has been established that 15 min after a single introduction of OSS with 0.7 mg/L ozone concentration, a transitory disruption of HEB permeability took place, which was manifested by partial reduction of interendothelial tight junctions, widening of intercellular gaps, swelling of the basement membrane and part of the astrocyte processes in the pericapillary area. Neural ultrastructure alterations were of the adaptive character in response to the effect of the oxidative factor, and were characterized by the increase of protein-synthesizing organelle activity. A day after OSS action, increase of HEB permeability grew, on day 14 the HEB structure was partly normalized (capillary endothelium had tight intercellular junctions), and by day 30 the HEB ultrastructure and microcirculation had been restored. The increase of ozone concentration to 3.5 mg/L in the intracarotidly introduced OSS also resulted in the rise of HEB permeability, however, its restoration by day 30 was not observed. Besides, alterations of the microcirculatory blood vessels, neuron ultrastructure, and glias were more pronounced.
Conclusion. A single intracarotid introduction of OSS with 0.7 mg/L ozone concentration increases HEB permeability over 1 day period, which can provide a more effective influence of preparations on the brain cells in all phases of the cell cycle.
The main conditions for chemical preparations to penetrate through an intact hematoencephalic barrier (HEB) are a small molecular size of the substance, the degree of its ionization, solubility in lipids [1, 2]. A number of chemical preparations do not meet at least one of these requirements, and therefore their efficacy in treating malignant tumors of the brain is not high. Brain tumors are supposed by some authors to be caused by the increase of cerebral vessel permeability to a various degree within the neoplasms, though full disrupture of HEB is not observed [3].
A real way for the preparations to reach the cells may be the increase of HEB permeability for them. Methods capable to entail a temporary HEB opening have been described in the literature: hyperthermia, pressure elevation in the cerebral vessels above 90 mm Hg, inhalation of gas mixture with an increased content of carbon dioxide, injection of histamine, calcium channel blockers, leukotriene C4, bradykinine, intracarotid introduction of hyperosmolar solutions of mannitol; physiological transport mechanisms are currently under study as well [4–8]. But the majority of them have some drawbacks, among which are serious complications and a short therapeutic window, i.e. the time during which the delivery of preparations to the tumor through the damaged HEB is facilitated [1]. Therefore, to improve the efficacy of preparation action, the development of new effective methods enabling long-lasting but reversible alterations of HEB permeability remains vital.
Free radical compounds are known to promote HEB impairment. The possibility of using ozone as a source of active oxygen forms for HEB permeability increase has been studied [9, 10].
The aim of the investigation was to assess experimentally the effect of ozonized saline solution with various concentrations of ozone on the dynamics of hematoencephalic barrier permeability.
Materials and Methods. The study was conducted on mature outbred Wistar rats (n=93) weighing 320±10 g. All principles of treating animals were observed according to the Order of the Ministry of Health of the Russian Federation No.708н of 23.08.2010 “On approval of principles of laboratory practice” and in accordance with ethical principles established by European Convention for the Protection of Vertebrata used for Experimental and other Scientific Purposes (the Convention was passed in Strasbourg, March 18, 1986, adopted in Strasbourg, June 15, 2006) and approved by Ethics Committee of Privolzhsky Federal Medical Research Center.
Three series of experiments were carried out, in which the sensorimotor zone ultrastructure of the animal brain cortex was studied. In control series I 0.9% saline solution (SS) was introduced intracarotidly, in series II it was an ozonized saline solution (OSS) with ozone concentration of 0.7 mg/L, and in series III the concentration of ozone in OSS was 3.5 mg/L. Ozone was bubbled through SS during 40 min using Kwazar unit (Kwazar, Russia). The concentration of the dissolved ozone in the SS was determined with the help of the IKOZH-5 analyzer of ozone in liquid media (Russia). OSS was introduced via a catheter into the common carotid artery at a rate of 1 ml/min in the volume of 1 ml per 300 g of animal body mass under intra-abdominal Nembutal anesthesia (35 mg/kg). After infusions, the carotid artery was bandaged. Euthanasia was performed by immediate decapitation under Nembutal anesthesia (45 mg/g).
For morphological examinations, the tissue of the rat sensorimotor cerebral cortex (according to Paxinos and Watson Atlas [11]) was excised on the side of OSS or SS infusion. Examinations were performed 15 min, 1, 14 and 30 days after OSS introduction. Transmission electron microscopy was chosen as a method of evaluation of the HEB state. Samples for electron microscopy were prepared according to the standard procedure: fixation with 2.5% solution of glutaraldehyde and 1% solution of osmic acid. Then samples were imbedded into the mixture of epoxy resins: araldite and epon 812. Ultrathin sections were prepared on Ultrcut ultratome (Reichert-Jung, Austria) and examined under the Morgagni 268D transmission electron microscope (FEI, USA). Morphometry was done using AnalySIS program.
The analysis of HEB permeability was assessed according to the state of astroglia, basement membrane, and intercellular junctions of capillary endothelium, as it is known that in the cerebral endothelium, forming HEB, intercellular tight junctions are a key morphofunctional element determining barrier, transporting and signaling functions. Their mobility and alteration of architectural organization make it possible to change HEB permeability in a short period of time [12, 13].
Data were statistically processed by Statistica 6.0 program using Mann–Whitney test for independent samples. Differences were considered significant at p<0.05.
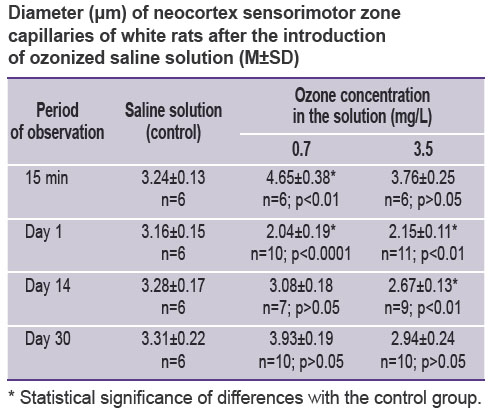
Results. Electron microscopy of the neocortex ultrastructure after intracarotid SS infusion did not reveal any changes of HEB permeability. The capillary diameter was equal to 3.24±0.13 μm (See the Table).
|
Diameter (μm) of neocortex sensorimotor zone capillaries of white rats after the introduction of ozonized saline solution (М±SD) |
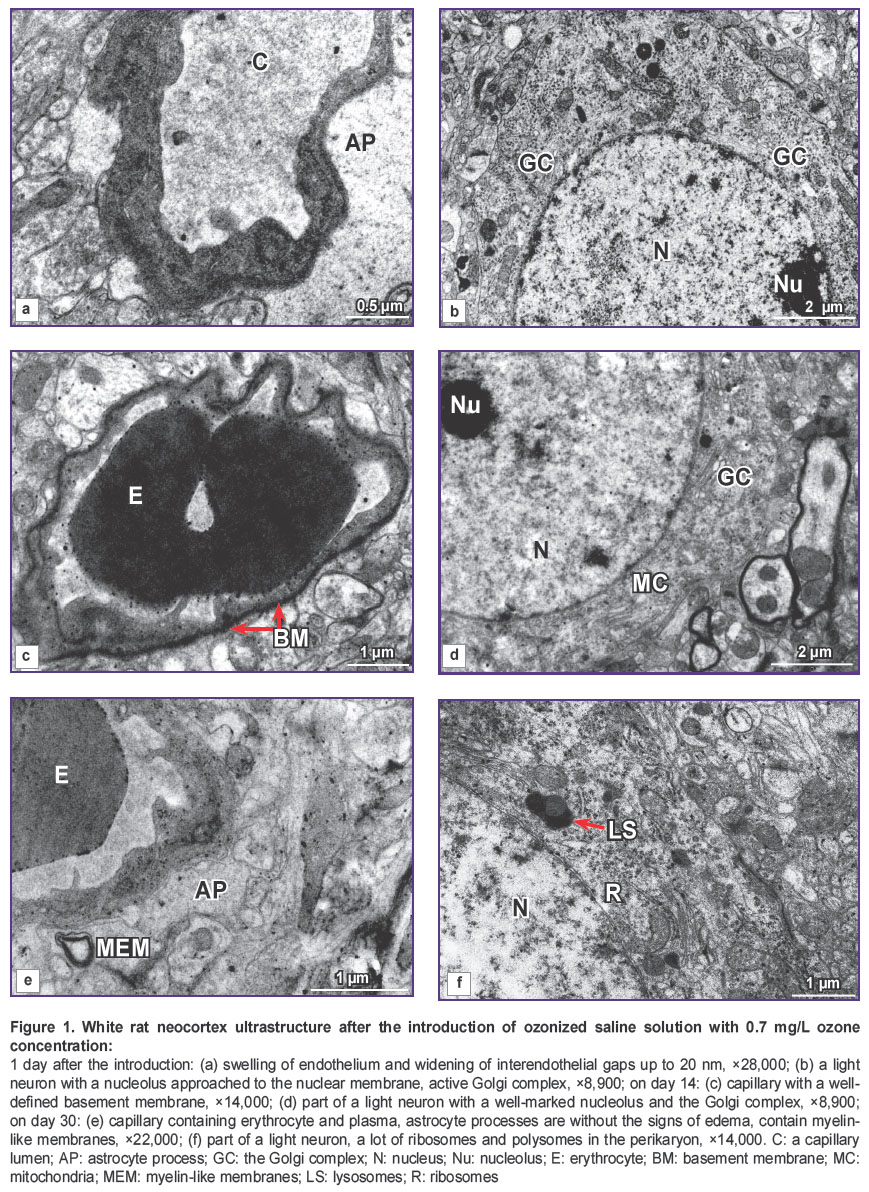
Focal swelling of the capillary endothelium and basement membrane was noted in the animal neocortex 15 min after the introduction of OSS with 0.7 mg/L ozone concentration. In the areas of swelling, reduction of interendothelial tight junctions was detected. Swelling of separate astrocyte processes in the pericapillary area was found. Capillaries had a wider lumen than in the control series (4.65±0.38 μm; p<0.001). In the vessel lumens, a fine-dispersed osmiofilic material and erythrocytes were seen. A considerable part of the light neurons contained a neucleolus, and in some of them its approaching to the karyolemma, widening of Golgi complex cisterns, insignificant invaginations of the nuclear membrane were detected.
A day after the introduction of OSS with 0.7 mg/L ozone concentration, the state of HEB testified to the increase of its permeability: the endothelium of the essential number of capillaries was swollen, had irregular contours, dark cytoplasm, unclear cellular membranes (Figure 1 (a)). In the cytoplasm, there were observed small vacuoles and pinocytic vesicles in the sublemmal area. Interendothelial gaps were widened (more than 20 nm). The basement membrane was swollen, wavy, dissociated in some places, fused over a considerable length. These zones surround, as a rule, swollen and enlarged processes of astrocytes, adjoining the capillary. Along with this, astrocytes of usual form without any signs of swelling were detected. Microcirculation also became worse: the majority of the vessels had a narrowed lumen (See the Table), contained plasma. In some vessels containing erythrocytes their adhesion to endothelium was observed. Light neurons had a well-defined ultrastructure: a nucleus contained euchromatin, in half of observations neurons had a well-marked nucleolus. In some of them a nucleolus was approached to karyolemma, which was accompanied by activation of the Golgi complex (Figure 1 (b)). In some neurons, a nucleus invagination was observed, in the cytoplasm of these neurons elongation and widening of the cisterns of the granular endoplasmic reticulum and the Golgi complex were noted. Mitochondria had a dense matrix, there were a lot of ribosomes and polysomes, primary and secondary lysosomes. Alongside with the light neurons, hyperchromic neurons were encountered.
On day 14 after the introduction of OSS with 0.7 mg/L ozone concentration, the electron microscopy showed restoration of the HEB structure in this series of animals: the capillary endothelium had predominantly recovered its usual appearance, and had dense intercellular junctions. In some endotheliocytes swollen mitochondria were detected. The basement membrane was clearly seen, and was swollen in some places (Figure 1 (c)). Astroglia was in the majority of cases without the signs of swelling. Microcirculation was noted to be restored (See the Table), fine-dispersed osmiofilic material and erythrocytes were found in the capillary lumens. In some cases, phagocytizing pericytes were identified. The light neurons were of usual appearance: nuclei contained euchromatin, nucleoli, the Golgi complexes (Figure 1 (d)), and sometimes swollen mitochondria. At the same time, single degenerative forms of neurons were encountered.
30 days after the introduction of OSS with 0.7 mg/L ozone concentration no impairment of HEB permeability was revealed. Perivascular space did not have signs of swelling. In single cases myelin-like membranes were noted in the astroglial processes (Figure 1 (e)). The great majority of vessels had a wide lumen and contained a finely dispersed osmiofilic material and erythrocytes. The light neurons were in various degree of activity and ultrastructure alteration: the majority of nuclei contained euchromatin, a lot of ribosomes and polysomes were observed in the cytoplasm, the Golgi complex was well-defined, primary and secondary lysosomes were encountered (Figure 1 (f)).
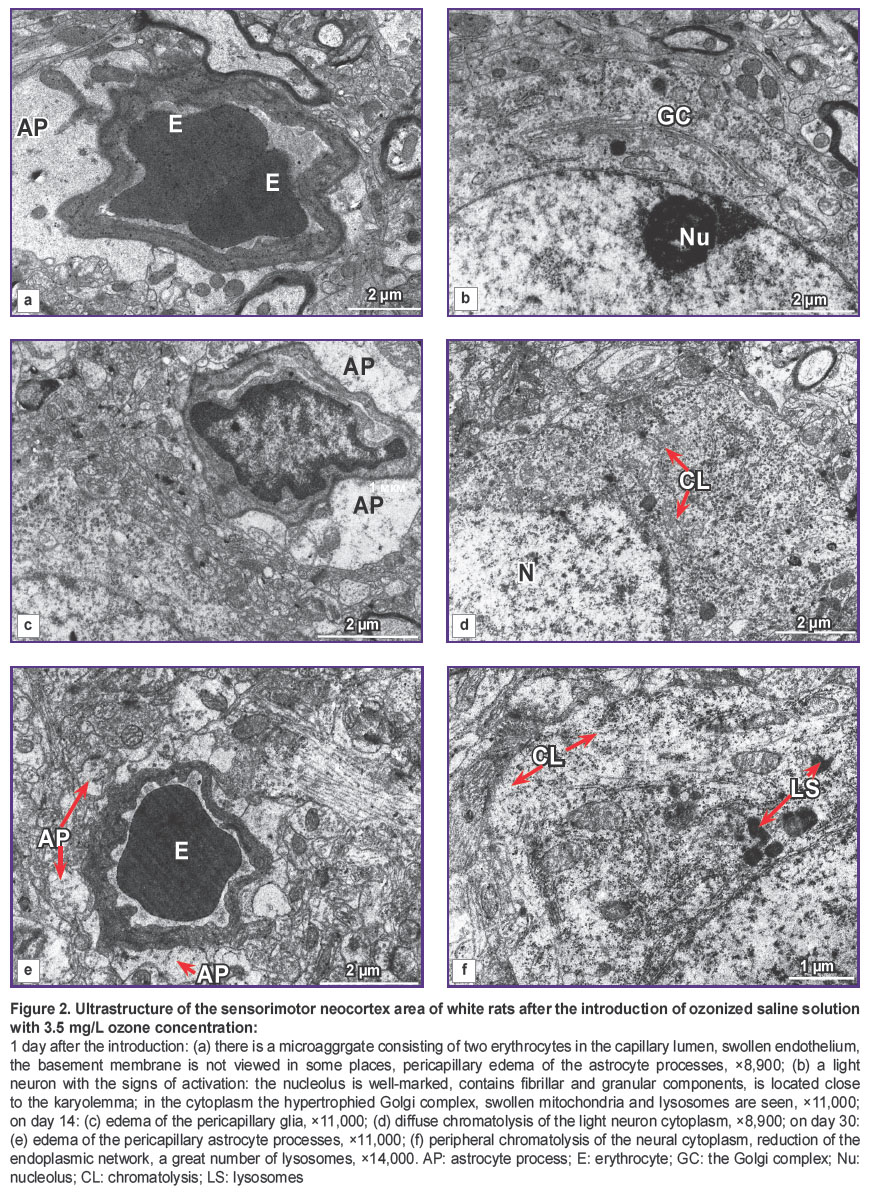
The analysis of capillary HEB ultrastructure of the sensorimotor cerebral cortex of Wistar rats 1 day after the introduction of OSS with 3.5 mg/L ozone concentration showed swelling of the capillary and basement membrane endothelium (Figure 2). Swollen mitochondria and the increased content of pinocytic vesicles were observed in endotheliocytes. Interendothelial gaps were widened (about 25 nm).
Increase of perivascular astroglia processes, their swelling and vacuolization were noted (Figure 2 (a)). In some instances, increased astrocyte processes surrounded the capillary-like a case, squeezing it. In the majority of the capillaries the diameter was considerably smaller than in the animals receiving the saline solution (p<0.01). Both ordinary blood and plasma, erythrocyte microaggregates were found in the capillaries, in single cases adhesion of erythrocytes to the endothelium was detected. Pericytes had the signs of phagocytosis. Some of the light neurons had an ordinary appearance, and some had the signs of minor diffuse chromatolysis (reduction of part of organelles, swelling of mitochondria, elongation of endoplasmatic reticulum cisterns, decrease of ribosomes and polysomes), neurons with the signs of protein-synthesizing organelle activation (nucleolus enlargement, its approaching the karyolemma, proliferation and hypertrophy of the Golgi complex, increase of ribosomes and polysomes) (Figure 2 (b)).
14 days after the introduction of OSS with 3.5 mg/L ozone concentration disruption of HEB permeability in the animals remained and was manifested by swelling of the capillary endothelium, basement membrane, lysis of some of its areas and wavy contour, edema of perivascular glia (Figure 2 (c)). The vessel lumen remained narrower than in the control group (p<0.01). 1/3 of the light neurons contained nucleoli, nuclei with invaginations, sometimes rather deep. Diffuse chromatolysis of separate neurons was noted (Figure 2 (d)).
On day 30 the HEB structure disruption was still preserved in these animals in the form of swelling of the endothelium, basement membrane, perivascular astroglia (Figure 2 (e)), focal fusion of the basement membrane, which caused alteration of the neural ultrastructure (Figure 2 (f)), i.e. the emergence of degenerative forms (cytoplasm chromatolysis, dark neurons).
Discussion. The analysis of HEB ultrastructure was carried out basing on the literature data, according to which enlargement of astrocyte processes adjoining the capillaries, irregular contour and nonuniform thickness of the basement membrane, increased number of pinocytic vesicles in the endothelium, violation of interendothelial contacts are the signs of HEB permeability disruption.
The results of the investigation showed that after a single introduction of OSS with 0.7 mg/L ozone concentration 15 min later impairment of HEB permeability took place, which manifested itself in partial reduction of interendothelial tight junctions, widening of intercellular gaps, swelling of the basement membrane and a portion of astrocyte processes in the pericapillary area. Junctions are dynamic structures capable to alter the size of intercellular gaps during some minutes by remodeling the cytoskeleton, and the pericapillary reaction of the astrocytes adjoining the capillaries signifies the increase of capillary wall permeability [14, 15]. Alterations of the neuron ultrastructure were of the adaptive character in response to the action of the oxidative factor, and were characterized by the increase of block-synthesizing organelle activity. A better microcirculation in comparison with the control animals was noted, as evidenced by the widening of capillary lumens.
A day after the OSS action, the increase of HEB permeability continued to grow: widening of interendothelial gaps up to 20 nm, pronounced swelling of pericapillary astroglia. As HEB permeability grew, microcirculation became worse. On day 14 the structure of HEB partially normalized: capillary endothelium had tight intercellular junctions. A slight swelling of astrocyte processes remained. Microcirculation was noted to be restoring. By day 30 the HEB ultrastructure and microcirculation had restored.
Increase of ozone concentration in OSS introduced intracarotidly up to 3.5 mg/L resulted in the increase of HEB permeability, though its restoration by day 30 was not observed. Besides, alterations of microcirculation, neuron and glia ultrastructure were more expressed.
Thus, intracarotid introduction of OSS with 0.7 mg/L ozone concentration causes a long-term (up to a day) transtory increase of HEB permeability with its partial restoration by day 14 and a full restoration by day 30. Temporary increase of HEB permeability has perspectives of a wide clinical application in neurooncology during brain tumor chemotherapy as well as in neurology for targeted delivery of drugs, which poorly penetrate through the intact HEB to the damaged brain foci.
Conclusion. A single intracarotid introduction of ozonized saline solution with 0.7 mg/L ozone concentration causes a long-term (up to a day) transitory increase of HEB permeability, which can provide a more effective action of pharmaceutical preparations on the brain cells in all phases of the cell cycle, improve the outcomes of treating brain tumors and a number of neurological diseases.
Study Funding and Conflicts of Interest. The work was not supported by any source, and there are no conflicts of interest related to this study.
References
- Blanchette M., Tremblay L., Lepage M., Fortin D. Impact of drug size on brain tumor and brain parenchyma delivery after a blood–brain barrier disruption. J Cereb Blood Flow Metab 2014; 34(5): 820–826, https://doi.org/10.1038/jcbfm.2014.14.
- Shilo M., Sharon A., Baranes K., Motiei M., Lellouche J.P., Popovtzer R. The effect of nanoparticle size on the probability to cross the blood-brain barrier: an in-vitro endothelial cell model. J Nanobiotechnology 2015; 13: 19, https://doi.org/10.1186/s12951-015-0075-7.
- Shamaev M.I. Izmenenie mozgovykh sosudov pri gliomakh polushariy golovnogo mozga. Avtoref. dis. … dokt. med. nauk [Change of cerebral vessels in gliomas of cerebral hemispheres. PhD Thesis]. Kiev; 1983.
- Chen Y., Liu L. Modern methods for delivery of drugs across the blood-brain barrier. Adv Drug Deliv Rev 2012; 64(7): 640–665, https://doi.org/10.1016/j.addr.2011.11.010.
- Kiviniemi V., Korhonen V., Kortelainen J., Rytky S., Keinänen T., Tuovinen T., Isokangas M., Sonkajärvi E., Siniluoto T., Nikkinen J., Alahuhta S., Tervonen O., Turpeenniemi-Hujanen T., Myllylä T., Kuittinen O., Voipio J. Real-time monitoring of human blood-brain barrier disruption. PLoS One 2017; 12(3): e0174072, https://doi.org/10.1371/journal.pone.0174072.
- Leuthardt E.C., Duan C., Kim M.J., Campian J.L., Kim A.H., Miller-Thomas M.M., Shimony J.S., Tran D.D. Hyperthermic laser ablation of recurrent glioblastoma leads to temporary disruption of the peritumoral blood brain barrier. PLoS One 2016; 11(2): e0148613, https://doi.org/10.1371/journal.pone.0148613.
- Li C.H., Shyu M.K., Jhan C., Cheng Y.W., Tsai C.H., Liu C.W., Lee C.C., Chen R.M., Kang J.J. Gold nanoparticles increase endothelial paracellular permeability by altering components of endothelial tight junctions, and increase blood-brain barrier permeability in mice. Toxicol Sci 2015; 148(1): 192–203, https://doi.org/10.1093/toxsci/kfv176.
- Patel M.M., Patel B.M. Crossing the blood-brain barrier: recent advances in drug delivery to the brain. CNS Drugs 2017; 31(2): 109–133, https://doi.org/10.1007/s40263-016-0405-9.
- Jung Y.S., Lee S.W., Park J.H., Seo H.B., Choi B.T., Shin H.K. Electroacupuncture preconditioning reduces ROS generation with NOX4 down-regulation and ameliorates blood-brain barrier disruption after ischemic stroke. J Biomed Sci 2016; 23: 32, https://doi.org/10.1186/s12929-016-0249-0.
- Rochfort K.D., Collins L.E., McLoughlin A., Cummins P.M. Tumour necrosis factor-α-mediated disruption of cerebrovascular endothelial barrier integrity in vitro involves the production of proinflammatory interleukin-6. J Neurochem 2016; 136(3): 564–572, https://doi.org/10.1111/jnc.13408.
- Paxinos G., Watson C. The rat brain in stereotaxic coordinates. 4th edition. San Diego: Academic Press; 1998.
- Ghehonin V.P., Baklaushev V.P., Yusubaliyeva G.M., Volgina N.E., Gurina O.I. Fundamental and applied aspects of the blood-brain barrier research. Vestnik Rossiyskoy akademii meditsinskikh nauk 2012; 8: 66–78.
- Erdő F., Hutka B., Dénes L. Function, aging and dysfunction of blood-brain barrier. Crossing the barrier. Orv Hetil 2016; 157(51): 2019–2027.
- Cabezas R., Avila M., Gonzalez J., El-Bachá R.S., Báez E., García-Segura L.M., Jurado Coronel J.C., Capani F., Cardona-Gomez G.P., Barreto G.E. Astrocytic modulation of blood brain barrier: perspectives on Parkinson’s disease. Front Cell Neurosci 2014; 8: 211, https://doi.org/10.3389/fncel.2014.00211.
- Liu X., Sui B., Sun J. Blood-brain barrier dysfunction induced by silica NPs in vitro and in vivo: involvement of oxidative stress and Rho-kinase/JNK signaling pathways. Biomaterials 2017; 121: 64–82, https://doi.org/10.1016/j.biomaterials.2017.01.006.