Visual Object Agnosia in Brain Lesions (Review)
Visual gnostic disorders are among the possible causes of disability in patients with brain lesions, but their prevalence and clinical significance in neurological practice are underestimated. This review gives insight into visual object agnosia as a manifestation of brain pathology.
Particular attention is paid to the present-day ideas of the neuroanatomical and neurophysiological basis of visual gnosis. Clinical variants of visual object agnosia, their morphological substrates, features of neuropsychological diagnosis and basic approaches to patient rehabilitation are described.
The unique possibilities of computer technologies for implementation of physical measurement principles, digital mapping and controlled optimization in diagnostic and rehabilitation processes, particularly, in visual object agnosia, are presented.
Special emphasis is placed on the necessity to develop standardized valid methods for diagnosing visual object agnosias to improve the ways of their correction in neurological practice.
Introduction
Visual gnosis (recognition) is the ability to recognize visually presented objects, i.e. to assign meaning to previously familiar visual stimulus when perceiving it as a whole or its separate parts [1, 2]. Accordingly, visual agnosia refers to inability of a person to recognize an object or a part of an object with the help of vision alone, while basic visual functions (visual acuity and visual fields, spatial contrast sensitivity, color vision), speech, memory and the ability to recognize objects by sound or tactile features remain intact [2–6].
Since visual agnosia is a monomodal disorder, clinical cases of patients having signs of gnostic function disorder of other modalities along with visual gnostic disorders should not be regarded as cases of true visual agnosia, according to some experts [3]. Visual agnosias can be observed in the clinical picture of many brain diseases and lesions, nevertheless, they are still among the least studied disorders in neurology [7]. However, the relevance of timely diagnosing visual gnostic disorders is determined by their negative impact on the quality of life of patients and the need for early correction [8].
Currently, many authors distinguish such types of visual agnosia as visual agnosia of objects and shapes, facial agnosia, topographical agnosia, letter agnosia [2, 8]. Visual agnosia of objects and shapes (object agnosia) remains the most understudied, it is characterized by inability to visually recognize complex objects or drawings and differentiate classes of stimuli, despite the intact basic visual functions [9]. Patients with visual object agnosia are unable to recognize previously familiar objects and unable to learn to identify new objects by their appearance alone [10]. Besides, such patients have reduced control over the accuracy of object recognition [2]. Visual object agnosia is likely to be accompanied by impaired recognition of familiar faces (prosopagnosia), less often letters and words (“pure” alexia without agraphia) [3, 11].
Classification of visual object agnosia
Object agnosias are divided into apperceptive and associative forms [5, 10, 12, 13]. Apperceptive object agnosia manifests itself by the inability to copy an object and find similarities/differences between objects [13–15]. Associative agnosia is characterized by impaired object identification due to the loss of knowledge about its meaning: the patient is able to draw the object, describe its parts and find similarities between different objects, but fails to recognize the object that has just been presented and drawn [14, 15].
Apperceptive visual agnosia comprises shape agnosia, transformational agnosia and integrative agnosia [5, 16, 17].
In shape agnosia, patients fail to recognize simple geometric shapes and, therefore, such elementary properties of objects as curvature and volume. They make mistakes in tests for recognition and comparison of objects and are unable to draw or copy the object seen [3]. Recognition is assisted by tracing the contour of the object with hands and touching it, visual perception is thereby translated into kinesthetic perception [10, 12]. A number of authors argue that this disorder is not purely agnostic and should be described as “pseudoagnosia” [12].
A patient with apperceptive integrative visual agnosia is unable to integrate the object details into a single unit and, therefore, fails to recognize the object and distinguish correct and incorrect images of real objects, although the patient can perceive separate object elements and copy the images in parts [10, 12, 16, 18].
Apperceptive transformational agnosia is the inability of a patient to recognize three-dimensional objects when looking at them from unusual visual angle and having to carry out their “mental turn” [10, 12, 19]. Some authors attribute this deficit to spatial agnosia. Other researchers believe that the term “spatial agnosia” can lead to confusion in this context as it gives a specialist the impression that errors of spatial information processing rather than impaired ability to perceive the same object from different visual angles underlie transformational agnosia [12].
Associative object agnosia is characterized by the fact that the patient is unable to identify the object and determine its semantic category, though he can analyze the structure of the object [18]. Some authors question the possibility of defining this disorder as true agnosia, since its mechanisms are closely related to selective visual memory impairment. It is believed that in patients with associative object agnosia, not only previously obtained knowledge about objects is impaired but also the possibility of acquiring new visual experience [10, 15]. Barton [10] suggested distinguishing two variants of the associative form of visual agnosia, depending on whether the patient had impaired access to the preserved traces of object imagery (semantic access agnosia) or visual object representation in memory was lost (full semantic agnosia).
Along with the two described forms of associative visual object agnosia, researchers discuss existence of category-specific agnosias defined as pathological conditions in which patients fail to recognize the stimuli related to specific categories, in particular, a group of living or non-living objects [10].
Neurophysiological and morphological basis of visual object gnosis
The nature of visual agnosia is most often explained relying on the ideas about normal processes of visual image formation, which comprise three levels [4]. The first (lowest) level involves visual stimulus processing and analysis of information about the simplest physical properties of the object. Eye structures, the corresponding pathways, the visual subcortical centers and the primary visual cortex (striate cortex) serve as the anatomical basis for these processes. The second (middle) level includes synthesis of visual information about the object properties, which is carried out involving the extrastriate cortex and is related to image formation at the psychological level [20–22]. At the third (highest) level there is polymodal information synthesis necessary for assigning a certain meaning to the object image; the neurophysiological basis of this synthesis is the activity of multimodal association areas of the cortex [4].
The hypothesis about two streams of extrastriate visual input, proposed by Leslie Ungerleider and Mortimer Mishkin, plays an important role in understanding the nature of object gnosis. According to the hypothesis, one of the streams goes from the primary visual cortex to the parietal cortex and is involved in processing the information about the spatial location and direction of movement of the object (the “where pathway”), while the second stream goes to the temporal cortex and is involved in recognition and identification of images (the “what pathway”).
Later, it was suggested that these streams had other functional differences: the ventral pathway was involved in processing the information necessary for stimuli perception and awareness of the surrounding world (vision for recognition), while the information important for action control and programming (vision for action) followed the dorsal pathway [5, 22–26]. The ventral pathway involved medial occipital-temporal structures, and the dorsal pathway involved lateral occipital-parietal structures [12, 25].
Currently, the ideas about isolatedness of visual information pathways in the extrastriate cortex have been challenged as there is evidence that both object recognition and actions with objects can be impaired regardless of lesion location in the ventral or dorsal pathway structures [5, 27, 28]. In this regard, a modified model of the above two-streams hypothesis has been proposed, according to which parallel and hierarchical coding of information about the object in the dorsal and ventral systems can be constantly modified within the framework of interaction between the two streams before convergence in the prefrontal cortex [14, 22, 28, 29].
To verify the morphological substrates of visual gnostic disorders, clinical data is compared to neuroimaging findings. The method of lesion-mapping involving statistical analysis of clinical picture dependence (in particular, visual agnosia manifestations) on lesion localization according to brain imaging data [30] is increasingly popular.
The lateral occipital complex, the posterior temporal cortex, the parahippocampal place area, and the fusiform face area are considered to be anatomical areas critically significant for visual object gnosis (object-sensitive areas) [14]. The lateral occipital complex is an area on the lateral surface of the occipital lobe [14]. It is activated when an object is presented from different view angles, but not when the object is resized or repositioned [18].
The posterior parts of the temporal cortex form the neurophysiological basis for linking the image of the object with its semantic meaning, while the parahippocampal place area is maximally activated in response to visual stimuli in the form of buildings and topographic landmarks [14, 31]. As for the fusiform gyrus, this area is activated mainly in response to faces, not objects [14].
Visual object agnosia has been described in cases of carbon monoxide poisoning, strokes, posterior reversible leukoencephalopathy syndrome, multiple brain metastases, herpetic encephalitis, posterior cortical atrophy, Creutzfeldt–Jakob disease [2, 9, 12]. When related to local brain lesions, object agnosia is more common in cases of bilateral temporal and occipital foci, though it is possible with underlying isolated lesion of the left or right hemisphere [2, 3, 12, 32].
Diagnosis of visual object agnosia
The complexity of diagnosing visual object agnosia is determined by the fact that in brain lesions it is often combined with other marked neuropsychological and neurological disorders concealing gnostic disorders [5, 33]. Another problem is lack of standardized methods for revealing gnostic disorders [12, 34]. On the other hand, timely clinical detection of object agnosia is greatly important, since it can be an early sign of neurodegenerative diseases for which neuroimaging methods of diagnosis have low sensitivity [33]. Accurate evaluation of the nature of agnosia allows developing an individual strategy of patient rehabilitation, improving the prognosis of his recovery [12].
Initial examination of a patient with suspected visual agnosia includes clinical examination involving assessment of elementary visual functions and simple visual stimuli perception. If these prove to be intact, the next step is evaluation of the ability to recognize objects [3, 16]. To do this, the patient is shown some familiar objects, asked to name them and describe their properties. The latter is necessary for differentiating agnosia from anomia: the patient with anomia cannot name the object, but correctly describes its function [2, 3]. Tasks on recognizing crossed-out images and superimposed figures are widely used [1, 16].
In patients with apperceptive visual object agnosia, recognition of overlapping images is dramatically reduced compared to images presented in isolation [16]. Patients with apperceptive integrative visual object agnosia are given tasks on distinguishing between real and meaningless object (created by adding or replacing parts) images, which represent the greatest difficulties [16].
Apperceptive visual shape agnosia (accompanied by impaired copying) is also diagnosed by giving tasks on drawing geometric shapes and letters [12]. To diagnose apperceptive visual transformational agnosia, there are tasks on recognizing objects demonstrated at atypical view angles and requiring mental spatial turns for recognition [12, 19].
Associative visual object agnosia is revealed by means of tasks on recognizing objects from various semantic categories and identifying their specific properties [12, 16].
Currently, there are few standardized tests to reveal visual object agnosia, which reduces reliability of object agnosia diagnosis [35].
Computer technologies for diagnosis and digital display of object agnosia
Traditional methods of diagnosis and rehabilitation of cognitive functions are adapted to cognitive-affective resources of human expert. The advantage of these methods is an active emotional-motivational component ensuring involvement of the individual in diagnostic and rehabilitation procedures (training). However, there are a number of important drawbacks: a limited space of features to describe the structure of the individual cognitive system, low detection accuracy, significant distortion of assessment results associated with cognitive-affective status of the expert. There is no possibility for objective digital mapping and controlled optimization of cognitive functions within conventional clinical strategies.
Development of computer technologies, virtual reality technologies, and software tools provides unique opportunities for making objective diagnosis and improving the efficiency of cognitive function correction [36]. There is technological background for implementing the basic principle of physical measurements in relation to human cognitive system: comparing like with like, the object with the standard. Information objects and event contexts of virtual computer environment can be considered as standards for measuring the properties of subjective cognitive space. In this case, measurement procedure can be reduced to formalized assessment of errors in recognition, management or reproduction of virtual standards. Measurement results are presented as digital cognitive maps providing objective display of the cognitive system of a particular individual in a wide range of cognitive parameters [37–39].
Present-day local and Internet-based software tools for cognitive assessment and rehabilitation (Lumosity.com, Cognifit.com, Wikium.ru, platform.apway.ru etc.) successfully ensure measuring and training perception, memory, attention, action speed, flexibility in relation to visual objects in various event contexts [40–43].
Testing is carried out in relation to visual objects of various semantics (object images, geometric shapes, letters, words, faces with a variety of emotional expressions) in a limited set of event contexts. Event contexts provide tasks on recognition, search and comparison of visual images [44–51]. Different types of recognition errors, response time, psychophysical detection and discrimination thresholds are measured. Different modifications of Wundt’s method (Figure 1 (a)), Stroop interference test (Figure 1 (b)), Gottschaldt figure test (Figure 1 (c)) are used as basic models for testing.
|
Figure 1. Examples of online implementation of basic test models (https://wikium.ru/science/techniques):
(a) Wundt’s method; (b) Stroop interference test; (c) Gottschaldt figure test |
In most computer-assisted diagnostic and rehabilitation simulators, tests are implemented in the form of exciting computer games. Feedback with digital assessment of trained functions and display of training history in the form of assessments time chart is a required element. Play and sport enthusiasm motivates for continued training using cognitive simulators. However, each game actualizes many cognitive processes, complicating differential diagnosis and correction of impaired cognitive module.
The expert system built on the ApWay platform (platform.apway.ru) developed in Privolzhsky Research Medical University (Nizhny Novgorod, Russia) can serve as an alternative to cognitive simulators available in the World Wide Web. The system provides an opportunity for digital mapping of cognitive functions in a wide feature space and provides a convenient interface for designing original user tests [52]. To date, the platform contains 350 scenarios that allow measuring certain cognitive modules in three target contexts: sensorimotor activity on a wide range of visual features and objects; object search; associations of multi-modal information images.
A unique infrastructure for testing the function of selecting visual objects from the background has been created in the ApWay environment using the original model of “computer campimetry” test [53] (Figure 2).
|
Figure 2. Variants of visual object semantics and localization on ApWay.ru Web platform |
Test scenarios based on this model can use visual objects with different semantics (object images, geometric objects, letters, words) localized in different areas of the screen. The user is assigned a task to display the object on the color background, to point at the icon for this object, hide the object (Figure 3).
|
Figure 3. Object image samples in computer campimetry method |
The same sequence of events for different background shades makes it possible to construct psychophysical function of color differentiation (Figure 4) which is a digital map of the subjective color space and displays color differentiation peculiarities of a particular individual. There is an opportunity for instrumental diagnosis of visual object agnosia irrespective of speech and mnestic functions.
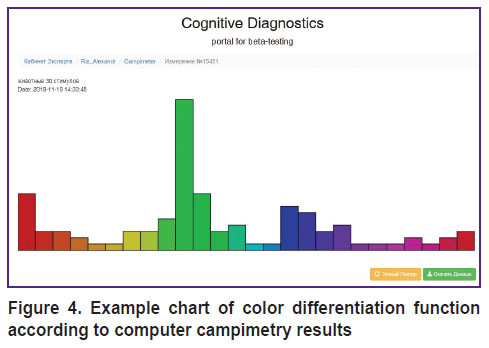
|
Figure 4. Example chart of color differentiation function according to computer campimetry results |
Every test on ApWay cognitive platform can be optimized for actualization of certain cognitive modules and provide both construction of personal digital cognitive maps and formation of individual cognitive training programs on their basis. A significant disadvantage of this technology is lack of a user-friendly interface for feedback and remote rehabilitation process monitoring.
Internet platforms providing cognitive simulators have been successfully tested for diagnosis and rehabilitation of dementia patients, post-stroke rehabilitation [54] and improving cognitive functions in cancer patients after chemotherapy [55].
Thus, there has been provided technological background for making cognitive diagnosis and rehabilitation reliable and objective. Template digital maps are developed taking into account basic features of information objects (spatial, temporal, quantitative, qualitative) and basic cognitive processes (feature selection, identification and classification of objects, selective attention, decision-making) for the main neuropsychological disorders, including visual object agnosia. Unique opportunities for personalized digitization of cognitive systems have been poorly realized so far. However, it should be admitted, there is active development in this direction.
Rehabilitation of patients with visual object agnosia
Spontaneous recovery is rare in visual object agnosia, increasing the importance of special training sessions with patients [2, 8]. Nevertheless, very little attention is paid to methods of restoration or compensation of visual gnosis, much less than to visual neglect correction issues, for example [7]. Indeed, in 2018, Heutink et al. found only seven works on rehabilitation of patients with visual object agnosia in scientific publications [8]. Research in this field is based only on certain clinical observations indicating that individual training of patients with visual agnosia can improve their ability to recognize objects and lead to certain generalization of positive effect [56].
Based on the results of 26-year observation of a patient with apperceptive integrative visual object agnosia, Humphreys [16] arrived at the conclusion that visual gnostic disorders can be partly compensated over time by involving the preserved dorsal pathway structures in recognition. It is recommended to train patients systematically in using compensatory techniques, such as conscious use of contextual, tactile, auditory clues and verbal description of objects [2, 8].
New principles of formal description of individual cognitive systems can lead to revising cognitive impairment classification and creating novel models of cognitive diagnosis and rehabilitation [57].
Conclusion
Visual gnostic disorders can cause significant limitations in the life of patients with brain lesions, but in practice, clinicians often underestimate them. It is necessary to develop standardized methods for diagnosing visual object agnosias and improve approaches to rehabilitation of patients with this disorder.
Study funding and conflict of interests. This study was not supported by any financial sources and the authors have no conflict of interests to disclose.
References
- Luria A.R. Osnovy neyropsikhologii [Foundations of neuropsychology]. Moscow: Izdatel’skiy tsentr “Akademiya”; 2013.
- Zihl J. Rehabilitation of visual disorders after brain injury. Psychology Press; 2010, https://doi.org/10.4324/9780203843253.
- Cooper S.A. Higher visual function: hats, wives
and disconnections. Pract Neurol 2012; 12(6): 349–357, https://doi.org/10.1136/practneurol-2011-000153. - Fundamental neuroscience. Squire L., Berg D., Bloom F.E., du Lac S., Ghosh A., Spitzer N.C. (editors). Elsevier; 2012.
- Haque S., Vaphiades M.S., Lueck C.J. The visual agnosias and related disorders. J Neuroophthalmol 2018; 38(3): 379–392, https://doi.org/10.1097/wno.0000000000000556.
- Martinaud O. Visual agnosia and focal brain injury. Rev Neurol (Paris) 2017; 173(7–8): 451–460, https://doi.org/10.1016/j.neurol.2017.07.009.
- Hanna K.L., Rowe F. Clinical versus evidence-based rehabilitation options for post-stroke visual impairment. Neuroophthalmology 2017; 41(6): 297–305, https://doi.org/10.1080/01658107.2017.1337159.
- Heutink J., Indorf D.L., Cordes C. The neuropsychological rehabilitation of visual agnosia and Balint’s syndrome. Neuropsychol Rehabil 2018; 1–20, https://doi.org/10.1080/09602011.2017.1422272.
- Barton J.J.S. Objects and faces, faces and objects…. Cogn Neuropsychol 2018; 35(1–2): 90–93, https://doi.org/10.1080/02643294.2017.1414693.
- Barton J.J. Disorders of higher visual processing. Handb Clin Neurol 2011; 102: 223–261, https://doi.org/10.1016/b978-0-444-52903-9.00015-7.
- Cavina-Pratesi C., Large M.E., Milner A.D. Visual processing of words in a patient with visual form agnosia: a
behavioural and fMRI study. Cortex 2015; 64: 29–46, https://doi.org/10.1016/j.cortex.2014.09.017. - Unzueta-Arce J., García-García R., Ladera-Fernández V., Perea-Bartolomé M.V., Mora-Simón S., Cacho-Gutiérrez J. Visual form-processing deficits: a global clinical classification. Neurologia 2014; 29(8): 482–489, https://doi.org/10.1016/j.nrleng.2012.03.023.
- Chechlacz M., Novick A., Rotshtein P., Bickerton W.L., Humphreys G.W., Demeyere N. The neural substrates of drawing: a voxel-based morphometry analysis of constructional, hierarchical, and spatial representation deficits. J Cogn Neurosci 2014; 26(12): 2701–2015, https://doi.org/10.1162/jocn_a_00664.
- Baars B.J., Gage N.M. Fundamentals of cognitive neuroscience: a beginner’s guide. Elsevier; 2013.
- Kolb B., Whishaw I.Q. Fundamentals of human neuropsychology. New York: Worth; 2015.
- Humphreys G. A reader in visual agnosia. Routledge; 2016, https://doi.org/10.4324/9781315668444.
Strappini F., Pelli D.G., Di Pace E., Martelli M. Agnosic vision is like peripheral vision, which is limited by crowding. Cortex 2017; 89: 135–155, https://doi.org/10.1016/j.cortex.2017.01.012.- Ptak R., Lazeyras F., Di Pietro M., Schnider A., Simon S.R. Visual object agnosia is associated with a breakdown of object-selective responses in the lateral occipital cortex. Neuropsychologia 2014; 60: 10–20, https://doi.org/10.1016/j.neuropsychologia.2014.05.009.
- Searle J.A., Hamm J.P. Mental rotation: an examination of assumptions. Wiley Interdiscip Rev Cogn Sci 2017; 8(6), https://doi.org/10.1002/wcs.1443.
- Angelucci A., Roe A.W., Sereno M.I. Controversial issues in visual cortex mapping: extrastriate cortex between areas V2 and MT in human and nonhuman primates. Vis Neurosci 2015; 32: E025, https://doi.org/10.1017/s0952523815000292.
- Kujovic M., Zilles K., Malikovic A., Schleicher A., Mohlberg H., Rottschy C., Eickhoff S.B., Amunts K. Cytoarchitectonic mapping of the human dorsal extrastriate cortex. Brain Struct Funct 2013; 218(1): 157–172, https://doi.org/10.1007/s00429-012-0390-9.
- Goodale M.A., Milner A.D. Two visual pathways — where have they taken us and where will they lead in future? Cortex 2018; 98: 283–292, https://doi.org/10.1016/j.cortex.2017.12.002.
- Merabet L.B., Mayer D.L., Bauer C.M., Wright D., Kran B.S. Disentangling how the brain is “wired” in cortical (cerebral) visual impairment. Semin Pediatr Neurol 2017; 24(2): 83–91, https://doi.org/10.1016/j.spen.2017.04.005.
- Goodale M.A. Separate visual systems for perception and action: a framework for understanding cortical visual impairment. Dev Med Child Neurol 2013; 55(Suppl 4): 9–12, https://doi.org/10.1111/dmcn.12299.
- Goodale M.A. How (and why) the visual control of action differs from visual perception. Proc Biol Sci 2014; 281(1785): 20140337, https://doi.org/10.1098/rspb.2014.0337.
- Foley R.T., Whitwell R.L., Goodale M.A. The two-visual-systems hypothesis and the perspectival features of visual experience. Conscious Cogn 2015; 35: 225–233, https://doi.org/10.1016/j.concog.2015.03.005.
- Rossetti Y., Pisella L., McIntosh R.D. Rise and fall of the two visual systems theory. Ann Phys Rehabil Med 2017; 60(3): 130–140, https://doi.org/10.1016/j.rehab.2017.02.002.
- Meichtry J.R., Cazzoli D., Chaves S., von Arx S., Pflugshaupt T., Kalla R., Bassetti C.L., Gutbrod K., Müri R.M. Pure optic ataxia and visual
hemiagnosia — extending the dual visual hypothesis. J Neuropsychol 2018; 12(2): 271–290, https://doi.org/10.1111/jnp.12119. - Takahashi E., Ohki K., Kim D.S. Dissociation and convergence of the dorsal and ventral visual working memory streams in the human prefrontal cortex. Neuroimage 2013; 65: 488–498, https://doi.org/10.1016/j.neuroimage.2012.10.002.
- Martinaud O., Pouliquen D., Gérardin E., Loubeyre M.,
Hirsbein D., Hannequin D., Cohen L. Visual agnosia and posterior cerebral artery infarcts: an anatomical-clinical study. PLoS One 2012; 7(1): e30433, https://doi.org/10.1371/journal.pone.0030433. - Ishii K., Koide R., Mamada N., Tamaoka A. Topographical disorientation in a patient with right parahippocampal infarction. Neurol Sci 2017; 38(7): 1329–1332, https://doi.org/10.1007/s10072-017-2925-6.
Rennig J., Cornelsen S., Wilhelm H., Himmelbach M., Karnath H.O. Preserved expert object recognition in a case of visualhemiagnosia . J Cogn Neurosci 2018; 30(2): 131–143, https://doi.org/10.1162/jocn_a_01193.- Cooper S.A., O’Sullivan M. Here, there and everywhere: higher visual function and the dorsal visual stream. Pract Neurol 2016; 16(3): 176–183, https://doi.org/10.1136/practneurol-2015-001168.
- De Vries S.M., Heutink J., Melis-Dankers B.J.M., Vrijling A.C.L., Cornelissen F.W., Tucha O. Screening of visual perceptual disorders following acquired brain injury: a Delphi study. Appl Neuropsychol Adult 2018; 25(3): 197–209, https://doi.org/10.1080/23279095.2016.1275636.
- Chiu E.-C., Wu W.-C., Chou C.-X., Yu M.-Y., Hung J.-W. Test-retest reliability and minimal detectable change of the test of
visual perceptual skills-third edition in patients with stroke. Arch Phys Med Rehabil 2016; 97(11): 1917–1923, https://doi.org/10.1016/j.apmr.2016.04.023. - Velichkovsky B.M., Solov’ev V.D. Komp’yutery, mozg, poznanie: uspekhi kognitivnykh nauk [Computers, brain, cognition: advances in cognitive sciences]. Moscow: Nauka; 2008. 293 p.
- Morrison G.E., Simone C.M., Ng N.F., Hardy J.L. Reliability and validity of the NeuroCognitive Performance Test, a web-based neuropsychological assessment. Front Psychol 2015; 6: 1652, https://doi.org/10.3389/fpsyg.2015.01652.
- Hardy J.L., Nelson R.A., Thomason M.E., Sternberg D.A., Katovich K., Farzin F., Scanlon M. Enhancing cognitive abilities with comprehensive training: a large, online, randomized, active-controlled trial. PLoS One 2015; 10(9): e0134467, https://doi.org/10.1371/journal.pone.0134467.
- Jiang T. Brainnetome: a new-ome to understand the brain and its disorders. Neuroimage 2013, 80: 263–272, https://doi.org/10.1016/j.neuroimage.2013.04.002.
- Feenstra H.E.M., Murre J.M.J., Vermeulen I.E., Kieffer J.M., Schagen S.B. Reliability and validity of a self-administered tool for online neuropsychological testing: the Amsterdam Cognition Scan. J Clin Exp Neuropsychol 2018 40(4): 253–273, https://doi.org/10.1080/13803395.2017.1339017.
- Fliessbach K., Hoppe C., Schlegel U., Elger C.E., Helmstaedter C. NeuroCogFX — a computer-based neuropsychological assessment battery for the follow-up examination of neurological patients. Fortschr Neurol Psychiatr 2006; 74(11): 643–650, https://doi.org/10.1055/s-2006-932162.
- Guimarães B., Ribeiro J., Cruz B., Ferreira A., Alves H., Cruz-Correia R., Madeira M.D., Ferreira M.A. Performance equivalency between computer-based and traditional pen-and-paper assessment: a case study in clinical anatomy. Anat Sci Educ 2018; 11(2): 124–136, https://doi.org/10.1002/ase.1720.
- Segalowitz S.J., Mahaney P., Santesso D.L., MacGregor L., Dywan J., Willer B. Retest reliability in adolescents of a computerized neuropsychological battery used to assess recovery from concussion. NeuroRehabilitation 2007; 22(3): 243–251.
- Tsotsos L.E., Roggeveen A.B., Sekuler A.B., Vrkljan B.H., Bennett P.J. The effects of practice in a useful field of view task on driving performance. Journal of Vision 2010; 10(7): 152–152, https://doi.org/10.1167/10.7.152.
- Crabb D.P., Fitzke F.W., Hitchings R.A., Viswanathan A.C. A practical approach to measuring the visual field component of fitness to drive. Br J Ophthalmol 2004; 88(9): 1191–1196, https://doi.org/10.1136/bjo.2003.035949 .
- Edwards J.D., Vance D.E., Wadley V.G., Cissell G.M., Roenker D.L., Ball K.K. Reliability and validity of useful field of view test scores as administered by personal computer. J Clin Exp Neuropsychol 2005; 27(5): 529–543, https://doi.org/10.1080/13803390490515432.
- Tombaugh T.N. TOMM, Test of Memory Malingering. North Tonawanda, NY: Multi-Health Systems; 1996.
- Korkman M., Kirk U., Kemp S. NEPSY. A developmental neuropsychological assessment. San Antonio, TX: The Psychological Corporation; 1998.
- Hooper H.E. Hooper Visual Organization Test (VOT) manual. Los Angeles, CA: Western Psychological Services; 1983.
- Conners C.K. Conners’ Rating Scale manual. North Tonawanda, NY: Multi-Health Systems; 1989.
- Greenberg L.M., Kindschi C.L., Corman C.L. TOVA test of variables of attention: clinical guide. St. Paul, MN: TOVA Research Foundation; 1996.
- Polevaya S.A., Mansurova (Yachmonina) Yu.O., Vetyugov V.V., Fedotchev A.I., Parin S.B. Osobennosti kognitivnykh funktsiy i ikh vegetativnogo obespecheniya pri narusheniyakh endogennoy opioidnoy sistemy. V kn.: XX Mezhdunarodnaya nauchno-tekhnicheskaya konferentsiya “Neyroinformatika–2018” [Specifics of cognitive functions and their vegetative provision in endogenous opioid system disorders. In: The XX International conference “Neuroinformatics–2018”]. Moscow: NIYaU MIFI; 2018; 2: 162–170.
- Polevaya S.A., Parin S.B., Stromkova E.G. Psikhofizicheskoe kartirovanie funktsional’nykh sostoyaniy cheloveka. V kn.: Eksperimental’naya psikhologiya v Rossii: traditsii i perspektivy [Psychophysical mapping of human functional states. In: Experimental psychology in Russia: traditions and perspectives]. Pod red. Barabanshchikova V.A. [Barabanshchikov V.A. (editor)]. Moscow: Izd-vo “Institut psikhologii RAN”; 2010; p. 534–538.
- Shatil E., Mikulecká J., Bellotti F., Bureš V. Novel television-based cognitive training improves working memory and executive function. PLoS One 2014; 9(7): e101472, https://doi.org/10.1371/journal.pone.0101472.
- Bray V.J., Dhillon H.M., Bell M.L., Kabourakis M., Fiero M.H., Yip D., Boyle F., Price M.A., Vardy J.L. Evaluation of a web-based cognitive rehabilitation program in cancer survivors reporting cognitive symptoms after chemotherapy. J Clin Oncol 2017; 35(2): 217–225, https://doi.org/10.1200/jco.2016.67.8201.
- Behrmann M., Peterson M.A., Moscovitch M., Suzuki S. Independent representation of parts and the relations between them: evidence from integrative agnosia. J Exp Psychol Hum Percept Perform 2006; 32(5): 1169–1184, https://doi.org/10.1037/0096-1523.32.5.1169.
- Polevaya S.А., Runova Е.V., Nekrasova М.М., Fedotova I.V., Bakhchina А.V., Kovalchuk А.V., Shishalov I.S., Parin S.B. Telemetry and information technologies in diagnosis of sportsmen functional state. Sovremennye tehnologii v medicine 2012; (4): 94–98.










