EEG Correlates of Tactile Perception Abnormalities in Children with Autism Spectrum Disorder
The aim of the investigation was to study the changes in EEG power and behavioral responses to C-tactile stimulation in typically developing (TD) children and children with autism spectrum disorder (ASD).
Materials and Methods. EEG to manually delivered tactile stimuli was recorded for 79 children (ASD=39, TD=40) aged 5 to 10 years. CARS scores were obtained for each participant immediately before the recording session. The study involved recording resting EEG in eyes open condition within 1–2 min and collecting EEG response to tactile stimuli delivered pseudo-randomly for 3 experimental conditions (stroking with a soft brush, stroking with a harsh brush, and stimulation with a spiked roller delivered to the outer side of right forearm, stroking velocity was within 2–5 cm/s). Behavioral responses obtained by video recording during the experiment were assessed and coded. Behavioral responses were classified into 5 patterns: 1) signs of relaxation (facial gesture and body posture); 2) signs of resistance, attempts to withdraw the hand; 3) negative emotions, crying, shouting; 4) positive emotions, smile, laughter; 5) looking at the hand being stimulated. EEG power in 18 narrow frequency bands with a bandwidth of 1 Hz in a range of 2–20 Hz was analyzed.
Results. The study revealed two types of response to tactile stimulation. The first type was not specific for particular tactile stimulation type, was accompanied by an increase in beta power (16–20 Hz) mainly in the left hemisphere and was more common in children with ASD. The second type of response was accompanied by an increase in frontal theta power (4–6 Hz) due to C-tactile system stimulation with a soft brush and was observed only in the TD children. The first type of response was accompanied by negative emotions and attempts to withdraw the hand, while the second type was characterized by relaxation.
Conclusion. The response of children with ASD to all types of tactile stimulation accompanied by an increase in beta power can be associated with both hypersensitivity and stress reaction of these children to the experimental situation. Selective response to C-tactile stimulation accompanied by an increase in frontal theta power has been found in the control group (TD) only. The results of this study can be useful for better understanding of hypersensitivity in children with ASD and gaining insight into the mechanisms of the disease.
Introduction
Autism spectrum disorder (ASD) is one of the most widespread developmental disorders. Although it is mostly seen as communication impairment, sensory perception abnormalities are also common in people with ASD [1]. As the nature of autism remains unknown, sensory perception gains academic interest as a possible source of its understanding. Tactile perception impairment is both widespread (up to 65% parents of children with ASD report tactile hypersensitivity [2]) and relatively understudied [3].
Lack of maternal contact was the first theory of autism [4]. It was criticized and later discarded, but it may have a new representation. In 1988, Johansson et al. [5] discovered C-tactile system in humans. C-tactile system consists of slow unmyelinated fibers whose afferents respond to soft touch and slow brushing, realizing affective touch, primarily, of a social kind. Since individuals with ASD are known to have a tendency towards evading such contacts [6], some researchers try to establish a link between C-tactile system and autistic behavior both in model animals and humans [7]. There is also a theory about the role of posterior superior temporal sulcus (pSTS) providing neurobiological connection between mental functions (social perception, action observation) impaired in people with ASD and the theory of mind [8]. pSTS is also involved in C-tactile processing [9], but its activation due to slow stroking is significantly less marked in individuals with ASD [10]. These data prove the theory of C-tactile system impairment as a part or even as a possible underlying mechanism for autistic brain development [9].
The aim of the investigation was to study the changes in EEG power and behavioral responses to C-tactile stimulation in typically developing children and children with autism spectrum disorder.
Materials and Methods
The relationship between С-tactile stimulation and autism was studied using electroencephalogram (EEG) findings. EEG is a noninvasive method, which makes it perfect for obtaining data in children and other individuals who have troubles controlling their behavior. Three types of tactile stimuli were selected: a soft brush for C-tactile stimulation [11], a harsh brush and a spiked roller as controls. We used video recording to analyze behavioral response to stimulation.
Participants. A total of 79 children participated in the study. Of them, 39 (mean age was 6.8±2.6 years, F/M ratio=10/29) had autism, as it had been confirmed by a licensed psychiatric clinic. They underwent a confirming assessment by a psychologist immediately before the procedure. The assessment included CARS [12], its mean score was 37.3, SD=5.9. The control group (typically developing (TD) children) included 40 children (mean age was 7.2±2.9, F/M ratio=16/24). Intellectual development was assessed with a nonverbal scale of Wechsler intelligence test (WPPSI) [13] as most children in the ASD group had language impairment. The resulting mean score in the ASD group was 102.9±2.8, in the TD group — 106.0±2.5.
Medical history of neurological or psychiatric diseases was the criterion of exclusion. Parents or legal guardians of each child gave their permission for the procedure. The study design was approved by the Ethics Committee of the Institute of Higher Nervous Activity and Neurophysiology of the Russian Academy of Sciences.
EEG recording. All the recordings were set in a study room of Equalize Psychology Center (Moscow). The participants had as much time to become acquainted with the room, as they needed to get calm.
Recording was done with a laptop with Encephalan (Medicom MTD, Russian) software Ag/AgCl electrodes, placed according to the International 10–20 System (Fp1, Fp2, F7, F3, Fz, F4, F8, T3, C3, Cz, C4, T4, T5, P3, Pz, P4, T6, O1, O2) [14]. The electrode impedances were less than 10 kΩ. Electrooculogram was performed in order to reduce artifacts.
The procedure. First, background EEG was recorded for a minute or two with open (if possible) eyes. Next, tactile stimulation was performed. The children received three types of tactile stimulation: stroking with a soft brush, stroking with a harsh brush and stimulation with a spiked roller. Stimulation was performed by a trained specialist with consistent speed (about 2–5 mm/s) on the outside surface of the right forearm for 10–15 s. Each type of stimulation was presented in a pseudorandom way three or four times.
Data processing. Eyes movement artifacts were cleaned out by the Encephalan software according to the electrooculogram data. Small intervals affected by muscle activity or any other artifacts were excluded manually using visual inspection. After artifact elimination, EEG was divided into sub-bands of 1 Hz bandwidth from 2–3 Hz to 19–20 Hz (18 sub-bands in total). Spectral power was determined with fast Fourier transform for each sub-band, each electrode and 4 types of stimulation (including background as zero stimulation).
Behavioral analysis. A videorecording of the procedure was later used for analysis of behavioral responses. Five response patterns were established during video analysis: 1) relaxation of facial gesture and body posture; 2) resistance, attempts to withdraw the hand; 3) negative emotions, crying, shouting; 4) positive emotions, smile, laughter — the total number of these behavioral reactions was counted during the applied stimulation; 5) looking at the hand being stimulated — percentage of time spent looking at their own hand divided by the total time of stimulation.
Statistical analysis. Statistical analysis was performed with Statistica 8.0. Logarithm of spectral power was found as a measure to normalize the data. Analysis of variance (ANOVA) was used to study the between-group differences.
Results
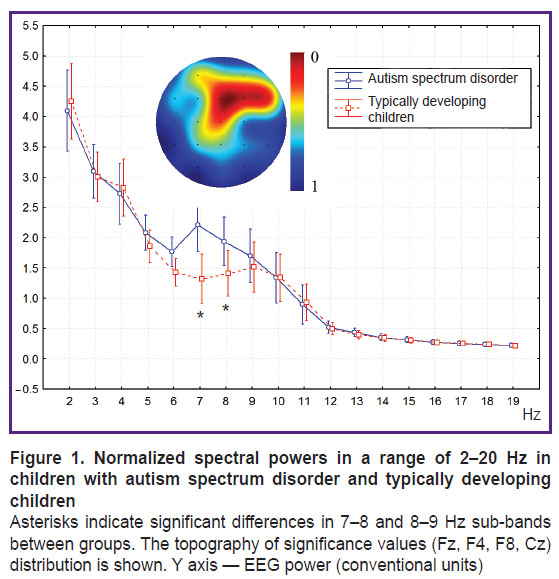
Background spectral power. Normalized spectral powers in the range of 2–20 Hz did not differ between groups, exclusive of 7–9 Hz range, where the children with ASD had their spectral power significantly increased compared to the TD group (F(1, 79)=5.6854, p=0.0200) (Figure 1).
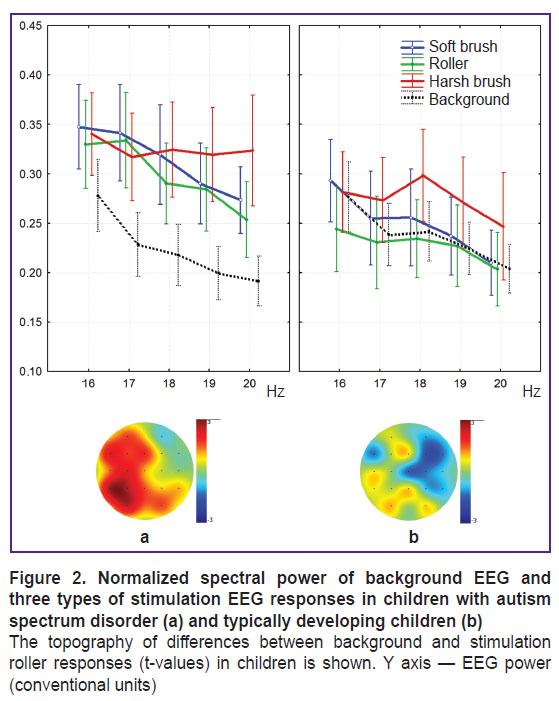
Spectral power during tactile stimulation. Unlike the TD children, ASD children have shown significant beta amplification in 16–20 Hz range in the left temporo-parieto-occipital regions (F(3. 237)=12.9401, p=0.0039) (Figure 2).
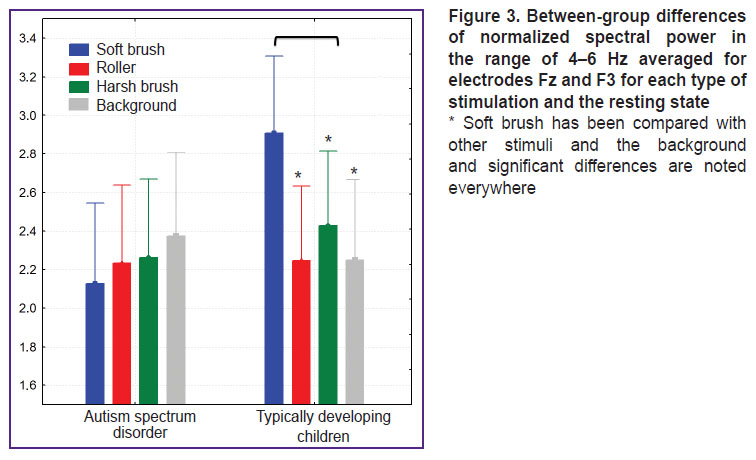
The soft brush elicited frontal theta (4–6 Hz) amplification in the TD group, but not in the ASD group (F(3. 237)=4.9105, p=0.0025). Other types of stimulation failed to elicit such reaction (Figure 3).
Behavioral responses to tactile stimulation. The children with ASD were more prone to negative emotional reaction to soft brush stimulation; they tried to evade it. The TD children were more prone to positive reactions. The harsh brush stimulation was also perceived more negatively by the ASD children, though they took more interest in the procedure as they watched it more closely than the TD children did. The spiked roller elicited quite different responses. Unlike the TD children, ASD children were interested in roller stimulation and showed positive emotions. The detailed analysis of behavioral responses and their differences between groups are shown in the Table.
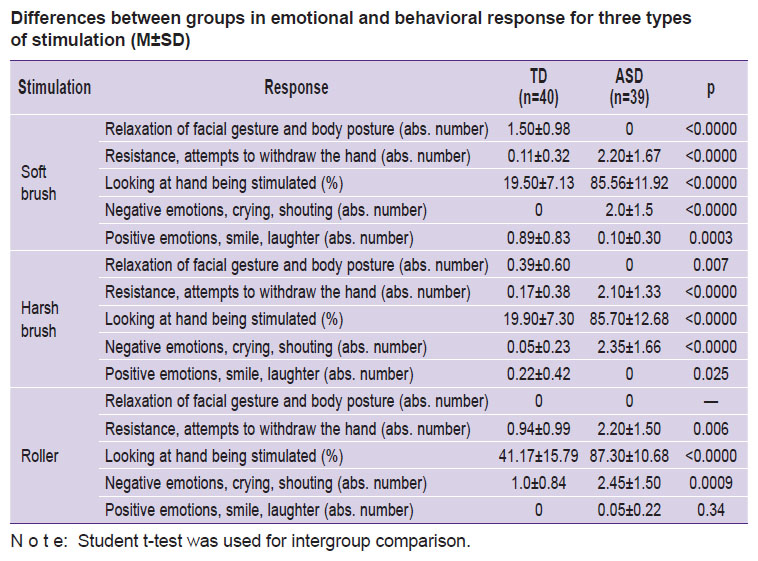
|
Differences between groups in emotional and behavioral response for three types of stimulation (M±SD) |
Correlations of behavioral responses and EEG. The correlation analysis of behavioral and EEG responses to the same tactile stimulation has shown a number of significant results.
Some emotional responses also correlated with EEG responses. For soft brush stimulation, beta differences (16–20 Hz) in left electrodes (Fp1, F3, Fz, P3, C3) correlated positively with negative emotional reaction (shouts, crying) to the touch (r>0.45; p<0.05) and negatively with relaxation (r<–0.41; p<0.05). For roller stimulation, leftward (Fp1, F3, P3, C3, О1) beta (16–20 Hz) differences correlated with negative emotional reaction (shouts, crying) to the touch (r>0.42; p<0.05). For harsh brush stimulation leftward (Fp1, F3, Fz, T3, P3, T5, C3, Pz, О1) beta (16–20 Hz) differences correlated with negative emotional reaction (shouts, crying) to the touch (r>0.42; p<0.05) as well as attempts to withdraw the hand (F3, Fz, T3, P3, C3, Pz).
The difference in theta sub-band (4–6 Hz) for electrodes F3 and Fz between a soft brush and a resting state conditions correlated with facial and body relaxation (r>0.48; p<0.05) and positive emotional response (r>0.41; p<0.05).
Discussion
Some of our findings have confirmed previous studies of peculiarities of resting state EEF in individuals with ASD. For example, group differences in background 7–9 Hz activity have confirmed previous results of relative theta amplification of background EEG in individuals with ASD [15]. Lack of other significant group background differences confirms correspondence of the groups in age [16] and intellectual development [17], allowing estimating the confidence of group differences in EEG responses to the tactile stimulation.
The chosen paradigm of tactile stimulation delivery made it possible to reveal some peculiarities of tactile perception in children with ASD. We found their beta spectral power to amplify in response to every type of tactile stimulation, while the TD children were less prone to such reaction. The behavioral responses were also different. The TD group demonstrated positive emotions in response to soft brush and roller stimulation and neutral to negative emotions towards harsh brush. The children with ASD had their responses almost similar to any type of stimulation; they were orienting, neutral or negative.
Beta rhythm amplification is a universal indicator of sensomotor activation and motion intention [18, 19], which are expected as a reaction to a touch, especially, unwanted or unexpected one. Beta amplification may also signalize a negative emotional reaction [20]. Thus, the response of the ASD children may be a reflection of their defensive reaction. This explanation is not the only one possible. Having analyzed the procedure video, we noticed that the ASD children were more prone to close watching their forearm being stimulated, assuming beta amplification to be a possible sign of cognitive activity enhancement in children with autism as shown in previous studies [21]. Beta amplification was already shown as an indicator of attention and concentration in children with ASD [22]. Thus, we assume that the elicited difference between groups was determined by emotional and cognitive response to the tactile stimulation in the ASD group. Results of previous studies confirm tactile hypersensitivity, especially, towards subjectively unpleasant stimuli in individuals with ASD [23–25]. A study of emotional responses towards tactile stimulation has shown that significant changes in beta rhythm spectral power correspond to the degree of emotional reaction expressiveness [26], which complies with our results.
The controls also differed from the target group by a specific reaction to soft brush stimulation: the frontal theta increase combined with visible body and face relaxation. This response agrees with known evidence of theta power changes as a correlate of a pleasant tactile sensation [27]. In particular, frontal theta amplification to tactile stimulation was described in previous studies [28]. Absence of said response to C-tactile stimulation in children with ASD has several possible explanations. Firstly, sensory impairments in people with ASD have great variability [29], which makes it hard to compare different study results and generalize conclusions. Secondly, frontal C-tactile response is developing with age, and the speed of its development also differs between subjects, impeding detecting of a pleasant tactile stimulus [30]. Finally, tactile hypersensitivity and defensiveness of children with ASD as well as inability to relax in a potentially dangerous experimental environment could also cause absence of theta amplification.
Conclusion
The response of children with ASD to all types of tactile stimulation accompanied by an increase in beta power can be associated with both hypersensitivity and stress reaction of these children to the experimental situation. Selective response to C-tactile stimulation accompanied by an increase in frontal theta power has been found in the control group only. The results of this study can be useful for better understanding of hypersensitivity in children with ASD and gaining insight into the mechanisms of the disease.
Study funding. The research was supported by the Russian Government contract, project 25.9502.2017/БЧ “A cross-cultural study of tactile communication: linguistic, social, and psycho-emotional aspects”.
Conflicts of interest. The authors declare no conflicts of interest related to this study.
References
- DiCicco-Bloom E., Lord C., Zwaigenbaum L., Courchesne E., Dager S.R., Schmitz C., Schultz R.T., Crawley J., Young L.J. The developmental neurobiology of autism spectrum disorder. J Neurosci 2006; 26(26): 6897–6906, https://doi.org/10.1523/jneurosci.1712-06.2006.
- Tomchek S.D., Dunn W. Sensory processing in children with and without autism: a comparative study using the short sensory profile. Am J Occup Ther 2007; 61(2): 190–200, https://doi.org/10.5014/ajot.61.2.190.
- Marco E.J., Hinkley L.B.N., Hill S.S., Nagarajan S.S. Sensory processing in autism: a review of neurophysiologic findings. Pediatric Research 2011; 69(5 Part 2): 48R–54R, https://doi.org/10.1203/PDR.0b013e3182130c54.
- Kanner L. Problems of nosology and psychodynamics of early infantile autism. Am J Orthopsychiatry 1949; 19(3): 416–426, https://doi.org/10.1111/j.1939-0025.1949.tb05441.x.
- Johansson R.S., Trulsson M., Olsson K.Å., Westberg K.-G. Mechanoreceptor activity from the human face and oral mucosa. Exp Brain Res 1988; 72(1): 204–208, https://doi.org/10.1007/bf00248518.
- McGlone F., Wessberg J., Olausson H. Discriminative and
affective touch: sensing and feeling. Neuron 2014; 82(4): 737–755, https://doi.org/10.1016/j.neuron.2014.05.001. - Baranek G.T. Autism during infancy: a retrospective video analysis of sensory-motor and social behaviors at 9–12 months of age. J Autism Dev Disord 1999; 29(3): 213–224, https://doi.org/10.1023/a:1023080005650.
- Yang D.Y., Rosenblau G., Keifer C., Pelphrey K.A. An integrative neural model of social perception, action observation, and theory of mind. Neurosci Biobehav Rev 2015; 51(8): 263–275, https://doi.org/10.1016/j.neubiorev.2015.01.020.
- Kaiser M.D., Yang D.Y., Voos A.C., Bennett R.H., Gordon I., Pretzsch C., Beam D., Keifer C., Eilbott J., McGlone F., Pelphrey K.A. Brain mechanisms for processing affective (and nonaffective) touch are atypical in autism. Cereb Cortex 2016; 26(6): 2705–2714, https://doi.org/10.1093/cercor/bhv125.
- Bennett R.H., Bolling D.Z., Anderson L.C., Pelphrey K.A., Kaiser M.D. fNIRS detects temporal lobe response to
affective touch. Soc Cogn Affect Neurosci 2014; 9(4): 470–476, https://doi.org/10.1093/scan/nst008. - Löken L.S., Wessberg J., Morrison I., McGlone F., Olausson H. Coding of pleasant touch by unmyelinated afferents in humans. Nat Neurosci 2009; 12(5): 547–548, https://doi.org/10.1038/nn.2312.
- Schopler E., Reichler R.J., DeVellis R.F., Daly K. Toward objective classification of childhood autism: Childhood Autism Rating Scale (CARS). J Autism Dev Disord 1980; 10(1): 91–103, https://doi.org/10.1007/bf02408436.
- Wechsler D. Weschler Intelligence Scale for Children: third edition manual. San Antonio, TX: The Psychological Corporation; 1991.
- Klem G.H., Lüders H.O., Jasper H.H., Elger C. The ten-twenty electrode system of the International Federation. The International Federation of Clinical Neurophysiology. Electroencephalogr Clin Neurophysiol Suppl 1999; 52: 3–6.
- Murias M., Webb S.J., Greenson J., Dawson G. Resting state cortical connectivity reflected in EEG coherence in individuals with autism. Biol Psychiatry 2007; 62(3): 270–273, https://doi.org/10.1016/j.biopsych.2006.11.012.
- Gasser T., Verleger R., Bächer P., Sroka L. Development of the EEG of school-age children and adolescents. I. Analysis of band power. Electroencephalogr Clin Neurophysiol 1988; 69(29): 91–99, https://doi.org/10.1016/0013-4694(88)90204-0.
- Fraga González G., Van der Molen M.J.W., Žarić G., Bonte M., Tijms J., Blomert L., Stam C.J., Van der Molen M.W. Graph analysis of EEG resting state functional networks in dyslexic readers. Clin Neurophysiol 2016; 127(9): 3165–3175, https://doi.org/10.1016/j.clinph.2016.06.023.
- Szurhaj W., Derambure P., Labyt E., Cassim F., Bourriez J.L., Isnard J., Guieu J.D., Mauguière F. Basic mechanisms of central rhythms reactivity to preparation and execution of a voluntary movement: a stereoelectroencephalographic study. Clin Neurophysiol 2003; 114(1): 107–119, https://doi.org/10.1016/s1388-2457(02)00333-4.
- Ohara S., Ikeda A., Kunieda T., Yazawa S., Baba K., Nagamine T., Taki W., Hashimoto N., Mihara T., Shibasaki H. Movement-related change of electrocorticographic activity in human supplementary motor area proper. Brain 2000; 123(6): 1203–1215, https://doi.org/10.1093/brain/123.6.1203.
- Güntekin B., Başar E. Event-related beta oscillations are affected by emotional eliciting stimuli. Neurosci Lett 2010; 483(3): 173–178, https://doi.org/10.1016/j.neulet.2010.08.002.
- Roohi-Azizi M., Azimi L., Heysieattalab S.,
Aamidfar M. Changes of the brain’s bioelectrical activity in cognition, consciousness, and some mental disorders. Med J Islam Repub Iran 2017; 31(1): 307–312, https://doi.org/10.14196/mjiri.31.53. - Cowan J., Markham L. EEG biofeedback for the attention problems of autism: a case study. In: 25th annual meeting of the Association for Applied Psychophysiology and Biofeedback. 1994; p. 12–13.
- Cascio C.J., Moana-Filho E.J., Guest S., Nebel M.B., Weisner J., Baranek G.T., Essick G.K. Perceptual and neural response to affective tactile texture stimulation in adults with autism spectrum disorders. Autism Res 2012; 5(4): 231–244, https://doi.org/10.1002/aur.1224.
- Cascio C.J. Somatosensory processing in neurodevelopmental disorders. J Neurodev Disord 2010; 2(2): 62–69, https://doi.org/10.1007/s11689-010-9046-3.
- Guclu B., Tanidir C., Mukaddes N.M. Tactile sensitivity of normal and autistic children. Somatosens Mot Res 2007; 24(1–2): 21–33, https://doi.org/10.1080/08990220601179418.
- Singh H., Bauer M., Chowanski W., Sui Y., Atkinson D., Baurley S., Fry M., Evans J., Bianchi-Berthouze N. The brain’s response to pleasant touch: an EEG investigation of tactile caressing. Front Hum Neurosci 2014; 8: 893, https://doi.org/10.3389/fnhum.2014.00893.
- von Mohr M., Crowley M.J., Walthall J., Mayes L.C., Pelphrey K.A., Rutherford H.J.V. EEG captures affective touch: CT-optimal touch and neural oscillations. Cogn Affect Behav Neurosci 2018; 18(1): 155–166, https://doi.org/10.3758/s13415-017-0560-6.
- Ackerley R., Eriksson E., Wessberg J. Ultra-late EEG potential evoked by preferential activation of unmyelinated tactile afferents in human hairy skin. Neurosci Lett 2013; 535: 62–66, https://doi.org/10.1016/j.neulet.2013.01.004.
- Crane L., Goddard L., Pring L. Sensory processing in adults with autism spectrum disorders. Autism 2009; 13(3): 215–228, https://doi.org/10.1177/1362361309103794.
- Björnsdotter M., Gordon I., Pelphrey K.A., Olausson H., Kaiser M.D. Development of brain mechanisms for processing affective touch. Front Behav Neurosci 2014; 8: 24, https://doi.org/10.3389/fnbeh.2014.00024.