Infrared Spectroscopy in Differential Diagnosis of Pulmonary Embolism
The aim of the study was to assess the effectiveness of infrared spectroscopy for verification of pulmonary embolism (PE) and a number of similar diseases.
Materials and Methods. Infrared spectroscopy was used to investigate blood serum of 19 healthy volunteers and 30 patients with intraoperatively confirmed PE as well as with chronic obstructive pulmonary disease (COPD) (n=10), pneumonia (n=10), tuberculosis (n=10), lung abscess (n=10) and lung cancer (n=10), acute disorder of cerebral circulation (ADCC) (n=10), ischemic heart disease (IHD) (n=10). Peak height ratios of absorption band were taken as diagnostic parameters (cm–1/сm–1): P1 — 1160/1165; P2 — 1165/1070; P3 — 1165/1150; P4 — 1165/1050; P5 — 1100/1050; P6 — 1025/1165. These parameters of IR spectrum are significant for the given nosology.
Results. The calculated indicators have demonstrated statistically significant difference of IR spectra parameters for the studied nosologies (p<0.001) even on the small samples supplementing each other and enabling step-by-step exclusion of lung abscess and pulmonary tuberculosis, COPD and pneumonia, cancer, IHD, ADCC, and PE.
The presented radar charts, built with consideration of the values of all peak height ratios of the absorption bands with diagnostically significant maxima, provided the possibility to visualize the IR profiles making the differentiation of PE and its clinical analogs not only more objective and reliable but also more explicit and compelling.
Conclusion. Infrared spectroscopy is a potentially effective method of PE differential diagnosis. Sample expansion will allow researchers to evaluate the sensitivity and specificity of this technique compared to the existing standard schemes of PE verification.
Introduction
A wide prevalence of pulmonary embolism (PE) associated with a high rate of risk factors for venous thrombosis, multiple complications, and significant probability of unfavorable disease outcome dictate the necessity to improve methods of its verification. The existing Russian and world standards prescribe to define the probability of PE in case of acute pain in the chest, loss of consciousness, breathlessness, and hemoptysis, however, symptoms often found in PE may also be similar to those manifested in dissecting aneurysm of the thoracic aorta, ischemic heart disease (IHD), pneumothorax, tuberculosis, obstructive, oncologic, purulent destructive diseases of the lungs, acute disorder of cerebral circulation (ADCC), and also in intercostal neuralgia with a highly prominent pain syndrome [1–4].
When attempting to evaluate the PE probability clinically, diagnostic errors occur rather often resulting in late rendering of specialized medical assistance, patient death, or formation of progressing complications also leading to fatal outcome [5–8]. In spite of the fact that chronic post-embolic pulmonary hypertension morbidity is 0.1–9.1% during the first two years after the episode of symptomatic PE [9], it is fixed in the International Registry that 74.8% of patients with persistent increased pressure in the pulmonary trunk had acute PE in the past history [10], and data are available on a wider prevalence of this syndrome: occurring in 5 people per 1 million population annually [11].
The development of chronic post-embolic pulmonary hypertension has been proved [12–14] to worsen not only patients quality of life but the disease prognosis as well, to require lifelong systemic therapy or operative intervention under extracorporeal circulation and hypothermia. Lethality and complications place a supplementary economic burden on the public health service and the state in general which, in particular, was clearly demonstrated in the recent investigations carried out in Russia and confirmed by the foreign authors [15–17].
The situation is still more aggravated by a frequent combination of the mentioned diseases since oncopathology and injury of bronchopulmonary and cardiovascular systems lead to hypercoagulation and blood flow deceleration and, in case of acute inflammatory reaction and a destructive process, to the damage of the vascular wall, i.e. to the formation of all three components of Virchow’s triad: an integral component of thrombus formation [18–20].
Methods and criteria for differential monopathology diagnosis have been proposed and are being developed, among which laboratory techniques are evolving most actively [21]. Spectral investigations are considered rather perspective in this respect. Infrared (IR) spectroscopy, in particular, is part of molecular optical spectroscopy studying absorption spectra and reflections of electromagnetic radiation in IR region, i.e. in the range of wavelengths from 10–6 to 10–3 m. This objective method does not practically require expensive reagents and consumables for its application and allows specialists to identify presence and dynamics of metabolite concentration showing the appropriate pathology of the specimen biochemical composition.
The aim of the investigation was to study the potentials of IR spectroscopy to verify pulmonary embolism and a number of similar clinical diseases.
Materials and Methods
The study was carried out jointly at the clinics of the Department of Hospital Surgery named after B.A. Korolyov and the Department of General Chemistry of Privolzhsky Research Medical University (Nizhny Novgorod) in two stages:
in the first stage, IR spectra of blood serum from 19 healthy volunteers and 30 patients with intraoperatively confirmed PE were obtained and compared;
in the second stage, IR spectra of blood serum were obtained and compared in PE and chronic obstructive pulmonary disease (COPD) (n=10), pneumonia (n=10), tuberculosis (n=10), lung abscess (n=10) and lung cancer (n=10), ADCC (n=10), IHD (n=10).
The work was done in compliance with the Declaration of Helsinki (2013) and approved by the Ethics Committee of Privolzhsky Research Medical University. Informed written consent was obtained from each patient.
5 ml of blood for the complex of investigations was collected from the cubital vein of each patient in the morning on their admission and centrifuged for 15 min at 1000 rpm. The separated serum (1.0 ml) was dried in the dry heat oven in the Petri dish at 25°С for 24 h. The solid serum residual was minced and suspended in liquid paraffin. The IR spectra of the dry blood serum were obtained using spectrophotometers SPECORD 75 IR and М80 (Carl Zeiss, Germany) with a photometric error of 0.2%.
At first, a height of absorption band peaks with maxima at 1165, 1160, 1150, 1100, 1070, 1050, 1025 cm–1 was determined. Then values of peak height ratios of the absorption bands (сm–1/сm–1) were calculated: P1 — 1160/1165; P2 — 1165/1070; P3 — 1165/1150; P4 — 1165/1050; P5 — 1100/1050; P6 — 1025/1165.
Results and Discussion
The mathematically processed results of the IR spectra of the blood serum taken from the healthy volunteers and PE patients were presented in the form of a radar chart (Figure 1).
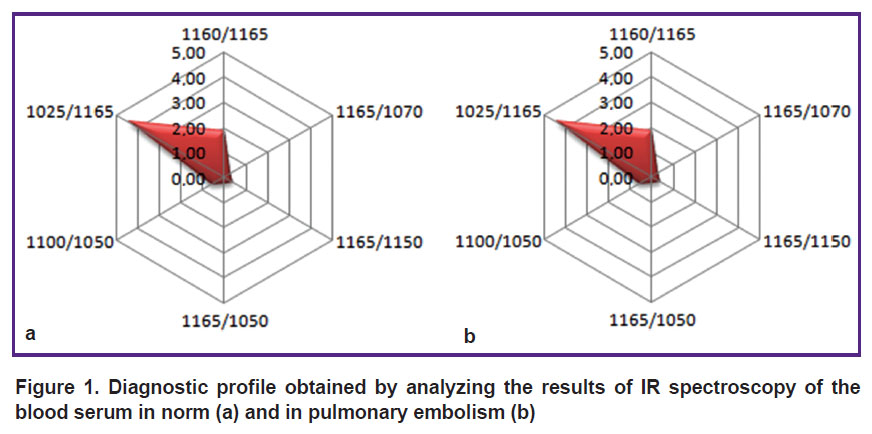
|
Figure 1. Diagnostic profile obtained by analyzing the results of IR spectroscopy of the blood serum in norm (а) and in pulmonary embolism (b) |
The obtained diagnostically significant IR spectra of the blood serum enabled us to develop the method of PE diagnosis based on the differentiation of serum molecular spectra in the range of 1200–1000 cm–1 wave numbers (in the IR range) [22].
Next, the IR blood serum spectra of the patients with COPD, pneumonia, tuberculosis, lung abscess, lung cancer, ADCC, IHD were obtained and transformed mathematically into the radar chart according to the same parameters. Thereafter, a comparative analysis of the radar charts of all mentioned nosologies with differentially diagnostic profile of PE was performed.
The comparison of 1100/1050 and 1160/1165 ratios demonstrates the differences of values across all examined diseases (p<0.001) and confirms indirectly the existence of common processes in the pathogenesis of all these diseases (Figure 2).
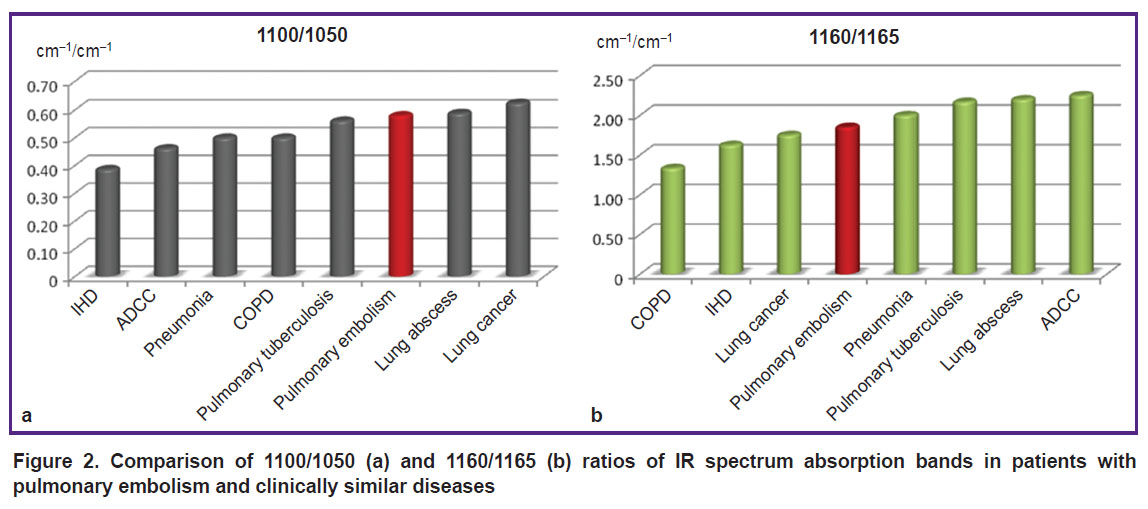
|
Figure 2. Comparison of 1100/1050 (а) and 1160/1165 (b) ratios of IR spectrum absorption bands in patients with pulmonary embolism and clinically similar diseases |
The difference of 1025/1165 ratio (Figure 3 (а)) was the most prominent for IR serum spectra of IHD, COPD, PE, and ADCC (p<0.001) and enabled the differentiation of lung abscess and pulmonary tuberculosis (p<0.001) but was less informative for COPD and pneumonia which would be preferably defined by 1165/1150 ratio, more typical in chronic bronchial obstruction (p<0.001) (Figure 3 (b)).
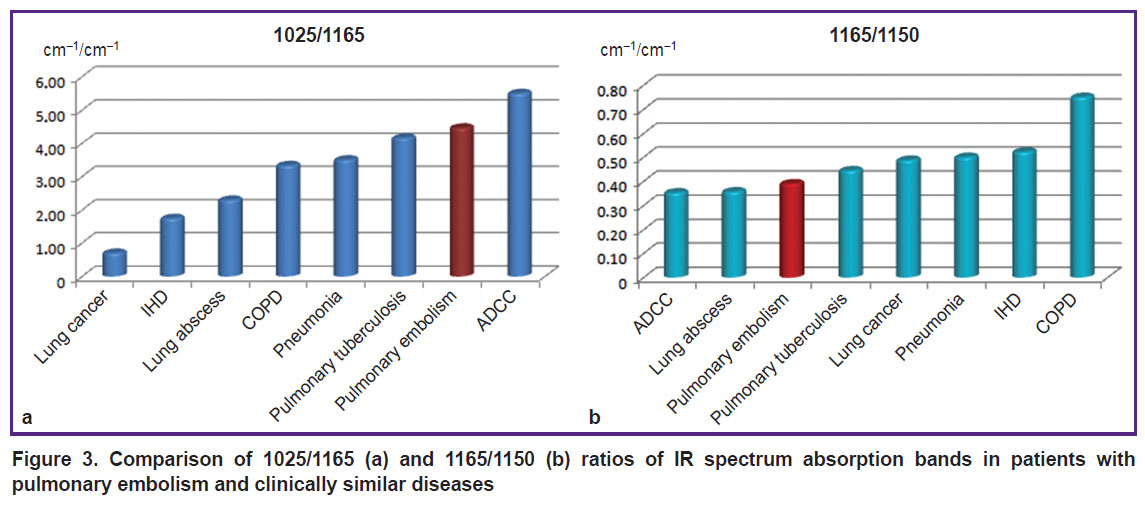
|
Figure 3. Comparison of 1025/1165 (а) and 1165/1150 (b) ratios of IR spectrum absorption bands in patients with pulmonary embolism and clinically similar diseases |
A high value of 1165/1050 ratio allowed us actually to suspect oncopathology (p<0.001) (Figure 4).
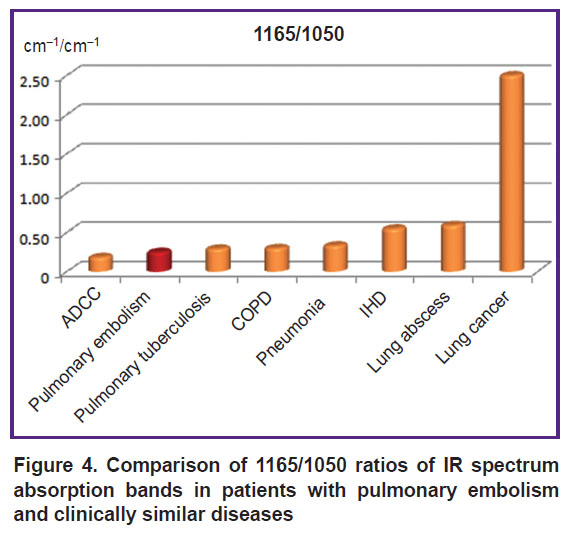
|
Figure 4. Comparison of 1165/1050 ratios of IR spectrum absorption bands in patients with pulmonary embolism and clinically similar diseases |
The 1165/1070 ratio showed also a maximal value in lung cancer (p<0.001) but unlike the previous charts demonstrated clearly the difference between COPD and pneumonia (p<0.001) and allowed us to differentiate PE from IHD (p<0.001) (Figure 5).
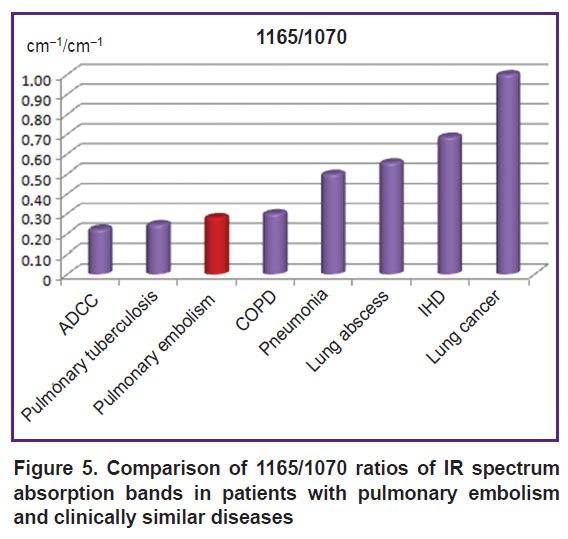
|
Figure 5. Comparison of 1165/1070 ratios of IR spectrum absorption bands in patients with pulmonary embolism and clinically similar diseases |
The chart built with consideration of all parameters became an illustrative differential diagnostic criterion for the diseases during verification of which the diagnostic errors are often encountered (Figure 6).
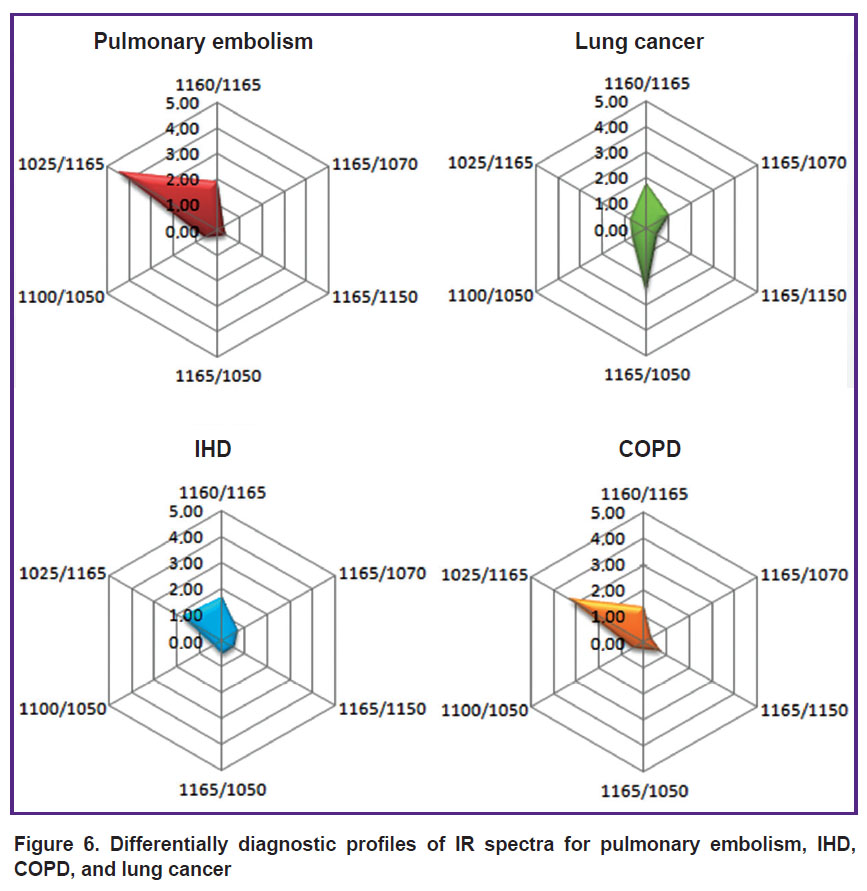
|
Figure 6. Differentially diagnostic profiles of IR spectra for pulmonary embolism, IHD, COPD, and lung cancer |
The current standards of PE diagnosis prescribe to define a D-dimer level, the normal values of which are considered a significant diagnostic criterion for the low risk of thromboembolism, however, its level rises in DIC syndrome, sepsis, and local inflammatory process, for example, in pneumonia, oncopathology, recent operative intervention, or in case of extensive hematoma, hepatic cirrhosis, and myocardial infarction [23, 24]. The diagnostically significant levels of the brain or pro-brain natriuretic peptides as well as troponin I or T are the markers of heart failure in the first place and will also rise in IHD, severe COPD, or bilateral pneumonia with the development of right ventricular insufficiency [25, 26], i.e. are considered to be criteria for the state severity but do not allow for the verification of its etiology [27].
In this work, the unique IR spectral characteristics (positions of the band maxima, their half-width, intensity) of molecules generated in the course of the pathologic metabolism initiated by PE constitute the basis of the differential diagnosis of PE and clinically similar diseases, therefore, the obtained values are distinguished by their high level individuality that makes them valuable for identifying the presence of organic compound combinations.
Despite a small sample volume for each illness and great heterogeneity of parameters within each group such as intensity of the main clinical symptoms and findings obtained by the laboratory and instrumental investigation methods, the calculated indicators have demonstrated a statistically significant difference between the IR spectra for the examined nosologies supplementing each other and enabling step-by-step exclusion of lung abscess and pulmonary tuberculosis, COPD and pneumonia, cancer, IHD, ADCC, and PE.
The presented radar charts built with consideration of the values of all peak height ratios of the absorption bands with diagnostically significant maxima provided the possibility to visualize the IR profiles having made the differentiation of PE and its clinical analogs not only more objective and reliable but also more explicit and compelling.
Conclusion
In the course of the pilot work, reliable results have been obtained demonstrating potential effectiveness of IR spectroscopy and, consequently, the appropriateness of its application in the differential diagnosis of pulmonary embolism. More extensive samples will allow researchers to evaluate the sensitivity and specificity of this technique compared to the existing standard schemes of pulmonary embolism verification.
Research funding. The work was not supported by any financial sources.
Conflicts of interests. The authors have no conflicts of interest to declare.
References
- Bagrova I.V., Kukharchik G.A., Serebriakova V.I., Konstantinova I.V., Kaputin M.Iu. The modern approaches to diagnostics of pulmonary embolism. Flebologiya 2012; 6(4): 35–42.
- Chuchalin A.G. Pul’monologiya [Pulmonology]. Moscow: GEOTAR-Media; 2014; 800 p. URL: http://www.rosmedlib.ru/book/ISBN9785970427712.html.
- Cherkasova N.A., Sergeeva E.V. Differentsial’naya diagnostika pri bolyakh v grudnoy kletke [Differential diagnosis for chest pain]. Moscow: GEOTAR-Media; 2009.
- Jolobe O.M.P. Pulmonary embolism in the differential diagnosis of right ventricular myocardial infarction. Am J Emerg Med 2019; 37(8): 1591–1592, https://doi.org/10.1016/j.ajem.2019.05.059.
- Fedchenko Ya.O., Protopopov A.V., Kochkina T.A., Stoliarov D.P., Konstantinov E.P., Gavrikov P.G. Efficacy of the treatment of pulmonary embolism depending on the time of patients’ admission. Mezhdunarodnyj zhurnal intervencionnoj kardioangiologii 2010; 21: 33–36.
- Fang M.C., Fan D., Sung S.H., Witt D.M., Schmelzer J.R., Williams M.S., Yale S.H., Baumgartner C., Go A.S. Treatment and outcomes of acute pulmonary embolism and deep venous thrombosis: the CVRN VTE Study. Am J Med 2019; 132(12): 1450–1457.E1, https://doi.org/10.1016/j.amjmed.2019.05.040.
- O’Connell C., Montani D., Savale L., Sitbon O., Parent F., Seferian A., Bulifon S., Fadel E., Mercier O., Mussot S., Fabre D., Dartevelle P., Humbert M., Simonneau G., Jaïs X. Chronic thromboembolic pulmonary hypertension. La Presse Médicale 2015; 44(12 Pt 2): e409–e416, https://doi.org/10.1016/j.lpm.2015.10.010.
- Tudela P., Mòdol J.M., Rego M.J., Bonet M., Vilaseca B., Tor J. Error diagnóstico en urgencias: relación con el motivo de consulta, mecanismos y trascendencia clínica. Med Clin (Barc) 2005; 125(10): 366–370, https://doi.org/10.1157/13079168.
- Lang I.M., Pesavento R., Bonderman D., Yuan J.X.-J. Risk factors and basic mechanisms of chronic thromboembolic pulmonary hypertension: a current understanding. Eur Respir J 2013; 41(2): 462–468, https://doi.org/10.1183/09031936.00049312.
- Pepke-Zaba J., Delcroix M., Lang I., Mayer E., Jansa P., Ambroz D., Treacy C., D’Armini A.M., Morsolini M., Snijder R., Bresser P., Torbicki A., Kristensen B., Lewczuk J., Simkova I., Barberà J.A., de Perrot M., Hoeper M.M., Gaine S., Speich R., Gomez-Sanchez M.A., Kovacs G., Hamid A.M., Jaïs X., Simonneau G. Chronic thromboembolic pulmonary hypertension (CTEPH): results from an international prospective registry. Circulation 2011; 124(18): 1973–1981, https://doi.org/10.1161/circulationaha.110.015008.
- Pepke-Zaba J., Jansa P., Kim N.H., Naeije R., Simonneau G. Chronic thromboembolic pulmonary hypertension: role of medical therapy. Eur Respir J 2013; 41(4): 985–990, https://doi.org/10.1183/09031936.00201612.
- Yandrapalli S., Tariq S., Kumar J., Aronow W.S., Malekan R., Frishman W.H., Lanier G.M. Chronic thromboembolic pulmonary hypertension: epidemiology, diagnosis, and management. Cardiol Rev 2018; 26(2): 62–72, https://doi.org/10.1097/crd.0000000000000164.
- Nižňanský M., Ambrož D., Prskavec T., Jansa P., Lindner J. Surgical treatment of chronic thromboembolic pulmonary hypertension. Vnitr Lek 2019; 65(5): 353–358.
- Jenkins D., Madani M., Fadel E., D’Armini A.M., Mayer E. Pulmonary endarterectomy in the management of chronic thromboembolic pulmonary hypertension. Eur Respir Rev 2017; 26(143): 160111, https://doi.org/10.1183/16000617.0111-2016.
- Chazova I.E., Martynyuk T.V., Valieva Z.S., Nakonechnikov S.N., Nedogoda S.V., Salasyuk A.S., Taran I.N., Gratsianskaya S.E. The economic burden of chronic thromboembolic pulmonary hypertension in Russian Federation. Terapevticheskij arhiv 2018; 90(9): 101–109.
- Grosse S.D., Nelson R.E., Nyarko K.A., Richardson L.C., Raskob G.E. The economic burden of incident venous thromboembolism in the United States: a review of estimated attributable healthcare costs. Thromb Res 2016; 137: 3–10, https://doi.org/10.1016/j.thromres.2015.11.033.
- Kirson N.Y., Birnbaum H.G., Ivanova J.I., Waldman T., Joish V., Williamson T. Excess costs associated with patients with chronic thromboembolic pulmonary hypertension in a US privately insured population. Appl Health Econ Health Policy 2011; 9(6): 377–387, https://doi.org/10.2165/11592440-000000000-00000.
- Grobben R.B., Frima C., Nathoe H.M., Leiner T., Kwakkel-van Erp J.M., van Klei W.A., Peelen L.M., van Herwaarden J.A. Pulmonary embolism after endovascular aortic repair, a retrospective cohort study. Eur J Vasc Endovasc Surg 2019; 57(2): 304–310, https://doi.org/10.1016/j.ejvs.2018.08.054.
- Hyde Congo K., Tomás A., Laranjeira Á., Afonso D., Fragata J. Type B aortic dissection with retrograde intramural hematoma and pulmonary embolism. Rev Port Cir Cardiotorac Vasc 2018; 25(1–2): 73–76.
- Ruiz-Artacho P., Trujillo-Santos J., López-Jiménez L., Font C., Díaz-Pedroche M.D.C., Sánchez Muñoz-Torrero J.F., Peris M.L., Skride A., Maestre A., Monreal M.; RIETE Investigators. Clinical characteristics and outcomes of patients with lung cancer and venous thromboembolism. TH Open 2018; 2(2): e210–e217, https://doi.org/10.1055/s-0038-1656542.
- Ateş H., Ateş İ., Bozkurt B., Çelik H.T., Özol D., Yldrm Z. What is the most reliable marker in the differential diagnosis of pulmonary embolism and community-acquired pneumonia? Blood Coagul Fibrinolysis 2016; 27(3): 252–258, https://doi.org/10.1097/mbc.0000000000000391.
- Gordetsov A.S., Krasnikova O.V., Nemirova S.V., Medvedev A.P. Diagnostic technique for pulmonary thromboembolism. Patent RU 2527346. 2014.
- Zhang Y., Zhou Q., Zou Y., Song X., Xie S., Tan M., Zhang G., Wang C. Risk factors for pulmonary embolism in patients preliminarily diagnosed with community-acquired pneumonia: a prospective cohort study. J Thromb Thrombolysis 2016; 41(4): 619–627, https://doi.org/10.1007/s11239-015-1275-6.
- Olson J.D., Adcock D.M., Bush T.A., de Moerloose P., Gardiner C., Ginyard V.R., Grimaux M., McMahan C.A., Prihoda T.J., Rico-Lazarowski A., Sales M., Stang L., Trumbull K., Van Cott E., Wissel T. Quantitative D-dimer for exclusion of venous thromboembolic disease; approved guideline. H-59A. Clinical and Laboratory Standards Institute; 2011.
- Zhang C., Jiang L., Xu L., Tian J., Liu J., Zhao X., Feng X., Wang D., Zhang Y., Sun K., Xu B., Zhao W., Hui R., Gao R., Yuan J., Song L. Implications of N-terminal pro-B-type natriuretic peptide in patients with three-vessel disease. Eur Heart J 2019; 40(41): 3397–3405, https://doi.org/10.1093/eurheartj/ehz394.
- Lin S.C., Tsai Y.J., Huang C.T., Kuo Y.W., Ruan S.Y., Chuang Y.C., Yu C.J. Prognostic value of plasma N-terminal pro B-type natriuretic peptide levels in pneumonia patients requiring intensive care unit admission. Respirology 2013; 18(6): 933–941, https://doi.org/10.1111/resp.12096.
- Mueller C., McDonald K., de Boer R.A., Maisel A., Cleland J.G.F., Kozhuharov N., Coats A.J.S., Metra M., Mebazaa A., Ruschitzka F., Lainscak M., Filippatos G., Seferovic P.M., Meijers W.C., Bayes-Genis A., Mueller T., Richards M., Januzzi J.L.J.; Heart Failure Association of the European Society of Cardiology. Heart Failure Association of the European Society of Cardiology practical guidance on the use of natriuretic peptide concentrations. Eur J Heart Fail 2019; 21(6): 715–731, https://doi.org/10.1002/ejhf.1494.










