Novel SOI-Biosensor Topology for the Detection of an Acute Myocardial Infarction Marker — Troponin I
A biosensor based on field-effect transistors on silicon-on-insulator structures (SOI-biosensor) is a high-potential device for detection of biological molecules, for instance, such as troponin I; the biosensor allows conducting label-free real-time analysis.
The aim of the study is the development of SOI-biosensor design for detection of acute myocardial infarction marker — troponin I.
A notable feature of this design was the integration of two grounding electrodes directly onto the biosensor surface, which effectively nullified the static potential of the liquid sample and minimized physical breakdowns of biosensor elements.
Materials and Methods. The highly specific anti-troponin I DNA aptamer was used as a receptor for specific detection of protein marker. Aptamer immobilization on the biosensor surface was carried out by physical adsorption. The analyzed range of target troponin I molecules concentration in the sample varied within 10–11 to 10–9 mol/L, mirroring clinical levels observed in myocardial infarction cases. During the experiment, a constant voltage of Vds=0.15 V was maintained.
Results. The developed SOI-biosensor successfully detected target troponin I molecules at a concentration of 10–11 mol/L. The detection process exhibited an effective time of approximately 200–300 s per sample. Moreover, analysis of the detection process revealed a noticeable decrease in current within the source-drain circuit, indicative of the negatively charged complex formed by troponin I and anti-troponin I DNA-aptamer at the “liquid sample–nanowire” phase interface.
Introduction
According to the World Health Organization, coronary heart disease (CHD) is among the major causes of death [1]. Impaired blood supply of myocardium can lead to myocardial infarction (MI) due to atherosclerotic damage to the arteries and the following necrotic processes in the heart tissue [2]. The degree of myocardium damage directly correlates with the time from the disease onset and restoration of the affected vessels patency, therefore timely and accurate diagnosis is of utmost importance for the patient prompt care.
Detection of MI-associated molecular markers is a diagnostic technique for the disease diagnosis. The major marker measured in the patient blood is troponin I (cTnI) [3]. This protein has a high clinical sensitivity to MI, as well as almost exclusive specificity to the heart tissue [4–8]. High cTnI levels in the blood flow indicate the death of myocardial contractile cells [6]. Usually, normal cTnI concentrations in the serum are less than 0.6 ng/mL (approximately 2.5×10–11 mol/L) [9, 10]. Minor myocardial damage can be seen at cTnI concentrations of 0.7 to 1.4 ng/ml, whereas necrotic myocardial damage is registered at protein concentrations over 1.5 ng/ml [9].
A modern high-potential diagnostic technique of troponin I detection is the use of a biosensor based on field-effect transistors on silicon-on-insulator structures (SOI-biosensor) [11–14], which allows conducting label-free real-time analysis. Moreover, it can serve as a basis for a portable tool for multicomplex analysis of various biological particles (proteins, viral particles, nucleic acids, etc.) [8, 11, 12, 15, 16].
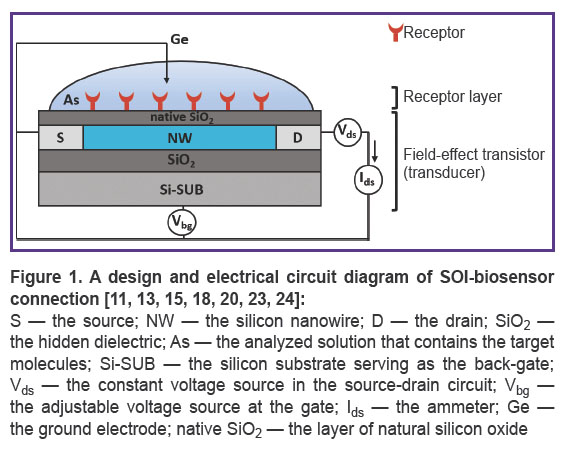
The SOI-biosensor has two main components: a receptor layer (contains antibodies, aptamers, enzymes, etc.) and a transducer — a field-effect transistor comprises of a silicon nanowire located between the source and drain electrodes [9]. The receptor layer ensures biospecific recognition of the target molecule as the interaction of the receptor and the target molecule generates a chemical or physical signal. This signal is further converted into an electrical output signal by a transducer [8, 11, 15, 17–19]. The following SOI-biosensors are widely used: devices with a ground electrode that is introduced directly “ontop” the analyzed liquid sample to eliminate random electrical potential caused by ions and charged molecules [15, 17, 18, 20–22].
Figure 1 shows a design and an electrical circuit diagram of SOI-biosensor connection [11, 13, 15, 18, 20, 23, 24].
Recently, aptamers were proposed as receptors [25–30]. These are short synthetic single-stranded deoxy- or ribo-oligonucleotides of a unique shape that can selectively bind to the corresponding target molecule [29–31]. Like antibodies, aptamers have high binding affinity.
The use of SOI-biosensor for detection of cTnI and other biological molecules is complicated by several issues, including the biosensor topology and design, optimal conditions for surface preparation, and the likelihood of target molecules adsorbtion to the biosensor surface [13, 15, 16, 20, 24, 32].
The aim of the study is the development of SOI-biosensor design for detection of the acute myocardial infarction marker — troponin I.
Materials and Methods
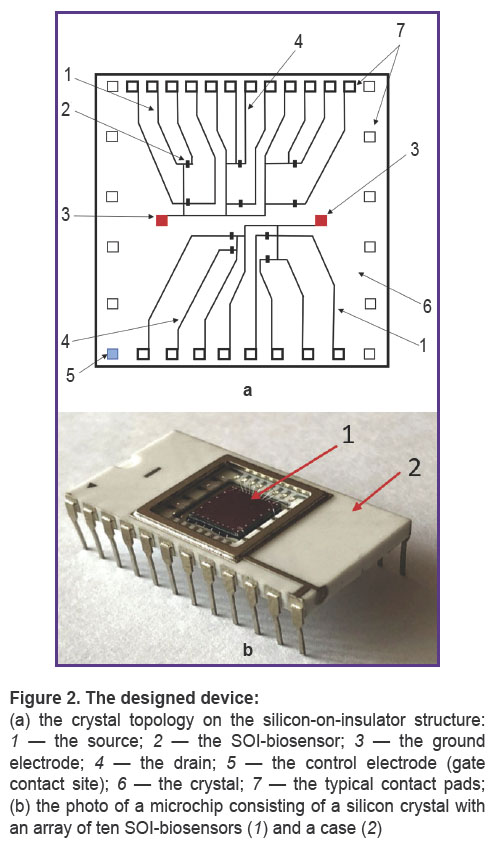
Structure of the SOI-biosensor. The SOI-biosensor design with two integrated ground electrodes on the microchip surface was developed. The crystal topology and SOI-biosensor design are shown in Figures 2 and 3, respectively. This non-standard product was manufactured at JSC “Novosibirsk Factory of Semiconductor Devices VOSTOK” (Russia).
An array of ten SOI-biosensors with n-type conductivity was formed on a 6×6 mm silicon crystal (Figure 2 (a)). The thickness of the silicon layer (Si-SUB) was 28–30 nm, the hidden dielectric layer (SiO2) — 200 nm. The rear side of the crystal was attached to the microchip. Typical contact pads were connected to the microchip body with aluminum conductors. The biosensor nanowire (NW) had the following geometric characteristics: height (H) — 20 to 30 nm, width (W) — 3 μm, and length (L) — 10 μm.
One output of each SOI-biosensor was connected to the ground electrode (3) and the source (1). The second output was connected to the drain electrode — 4 (see Figure 2 (a)).
SOI-biosensors were connected to a constant voltage source — Vds (see Figure 3). The biosensor operating mode was Vds=0.15 V.
Materials. The following materials were used in the study: sodium chloride (NaCl), potassium chloride (KCl), disodium hydrogen phosphate (Na2HPO4), potassium dihydrogen phosphate (KH2PO4), magnesium chloride (MgCl2), ethanol (C2H5OH) produced by Sigma-Aldrich (USA); recombinant cardiac troponin I (cTnI) produced by HyTest (Finland).
A highly specific anti-troponin I DNA aptamer (TnAp12t2) (5’-GGAAGACAAGACATCGGGAGGGAGG GAGGGCAGTCTAGTCTCATGTGTTTCCAT GGTTC-3’) was selected by SELEX using bioluminescent reporters to monitor the DNA libraries enrichment and assess the resulting candidate sequences affinity [33]. The dissociation constant (KD) of the “troponin I + anti-troponin I DNA aptamer” complex was 6×10–9 mol/L [33].
Before the experiment, the aptamer was thermally denatured at 90°C for 5 min in the binding buffer (0.15 mol/L NaCl, 50 mmol/L K-Na phosphate buffer (pH 7.0) and 1 mmol/L MgCl2). Then, the aptamer was renatured at room temperature for 15 min. TnAp12t2 and cTnI solutions were diluted with the distilled water (pH 5.9) immediately before the experiment to decrease the ionic strength and conductivity of the solutions [23, 34].
Measurement. The SOI-biosensor surface was modified to create a receptor layer by physical adsorption [35]. At the initial stage, the biosensor surface was washed with 96% ethanol and then with distilled water. Further, 5 μl of TnAp12t2 (CA=10–8 mol/L) was applied to the surface and incubated for several minutes [36]. Biosensor signals were continuously recorded (in real time) throughout the experiment. After the aptamer was added and the signal was stabilized, 5 μl of cTnI was added to the surface in the concentration of
CT=10–11–10–9 mol/L.
The SOI-biosensor signal was a change in the current in the source–drain circuit (Ids) during the adsorption of biological molecules, such as aptamers or the troponin I + anti-troponin I DNA aptamer complex, onto the nanowire surface. The current was measured using the PXIe 4163 ammeter (National Instruments, USA). The voltage in the source–drain circuit (Vds=0.15 V) was hold constant using the PXI 4135 device (National Instruments, USA). The voltage supplied to the sensor gate was selected within Vbg=0–30 V. The data received was visualized as the time dependence of the source–drain current — Ids(t).
Results
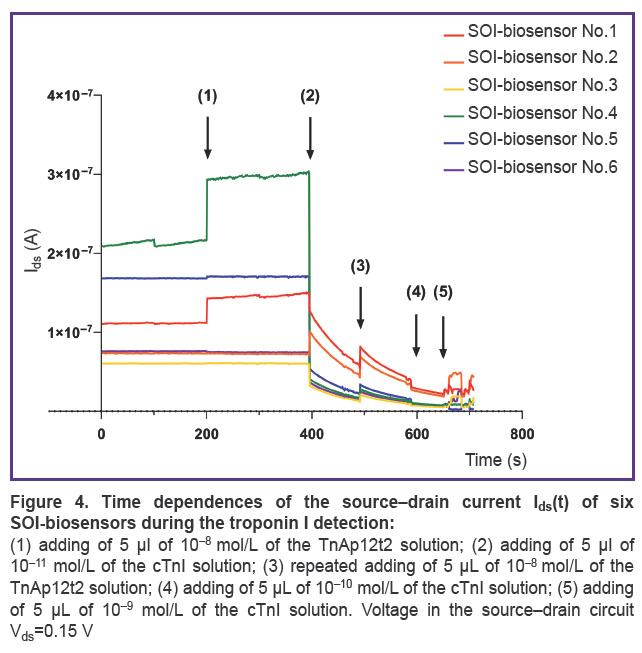
The SOI-biosensor time dependences of the source–drain current Ids(t) are demonstrated in Figure 4. It was found that six SOI-biosensors changed the current during the experiment. The other four SOI-biosensors operated in the cut-off mode (the fully closed mode) under specified experiment conditions, thus their Ids(t) curves are not shown in the figure.
The initial biosensor currents differed when the devices were turned on (see Figure 4). The recorded current values covered a wide range: from Ids=2.1×10–7 A (SOI-biosensor No.4, green line) to Ids=6.1×10–8 A (SOI-biosensor No.3, yellow line). During the idle mode (having no biological samples on the biosensor surface) in the time range of 0–200 s, the majority of SOI-biosensors demonstrated a stable Ids value, except for SOI-biosensors No.1 (red line) and No.4 (green line), which had a negligible and a noticeable current drift, respectively.
Adding 5 μl of TnAp12t2 solution (CA=10–8 mol/L) to the crystal surface at point (1) resulted in the current increase only in SOI-biosensors No.1 and No.4. Such a reaction of biosensor with the n-type conductivity was due to an increase in the number of charge carriers, here — electrons, in nanowire, indicating the presence of a positive effective electric charge at the liquid sample–nanowire phase interface. As the buffer solution had no impact on the current values, the positive charge was associated with the aptamer adsorption. Possibly under experimental conditions (pH 5.9), the positive effective electrical charge of the aptamer might be due to the protonation of adenine (pK ~3.5), cytosine (pK ~4.2), or guanine (pK ~2.1) [37–41]. The experimental results of the aptamer adsorption impact on the current value of the SOI-biosensor were consistent with the results of Farrow et al. [42].
A minor current drift was seen for SOI-biosensors No.5 (blue line) and No.6 (purple line). On the other hand, SOI-biosensors No.2 (orange line) and No.3 (yellow line) had no significant differences compared to the current in the idle mode.
Signal stabilization was recorded in the interval between points (1) and (2). However, SOI-biosensors No.1 and No.4 demonstrated a minor current drift, which indicated dynamic processes on their surface. The values of the remaining biosensors remained unchanged.
Adding 5 μl of the cTnI solution (C1=10–11 mol/L) to the crystal surface at point (2), which contained TnAp12t2 molecules, resulted in a sharp decrease in the Ids values in SOI-biosensors No.1, No.3–6 in the range from 10–1 to 3.8 A. This was an indicator of a negative effective electric charge at the liquid sample–nanowire phase interface. As the isolated troponin molecule (pI=9.3) under the experiment conditions had a positive electrical charge, one could conclude that the resulting “troponin I + anti-troponin I DNA aptamer” complex had a negative effective electrical charge at the phase interface [42–44].
Only the Ids of SOI-biosensor No.2 increased by 3×10–8 A. An increase in the Ids values of the SOI-biosensor with n-type conductivity was possible when a positive electric charge formed on the surface of the nanowire. The results of the experiment and literature data [42–44] provide that an increase in the Ids values can be caused by adsorption of the aptamer (for example, at point (1) in Figure 4) or an isolated troponin molecule (pI=9.3).
The Ids values of all SOI-biosensors demonstrated a general trend to change in the time range between points (2) and (3).
At point (3), the TnAp12t2 solution (CA=10–8 mol/L) was added to the crystal surface, which resulted in the increase in current of all SOI-biosensors. A subsequent introduction of 5 μl of the cTnI solution at a concentration of C2=10–10 mol/L at point (4) led to a decrease in the Ids values of all six SOI-biosensors due to formation of new “troponin I + anti-troponin I DNA aptamer” complexes. During this time range, the biosensors operated in the cut-off mode, which was aligned with their low sensitivity. Therefore, the changes in the Ids values registered at points (3) and (4) were less significant than those seen at points (1) and (2).
Addition of 5 μl of the cTnI solution (C3=10–9 mol/L) at point (5) resulted in unstable operation of SOI-biosensors due to accumulation of a large number of molecules on the biosensors surface.
The spread of the Ids values seen for the SOI-biosensors may be related to the chip manufacturing process, which affects biosensor sensitivity. This assumption is based on the spread of the Ids values of six SOI biosensors at devices turn-on point and in the idle mode, as well as when the four SOI-biosensors operate in the cut-off mode.
Discussion
SOI-biosensor is a high-potential analytical device for label-free detectionof the target molecules. It has high sensitivity and can record a signal in real time [11]. However, there are specific factors that complicate the SOI-biosensor application — for instance, the topology and design of the biosensor. In this study, we used a SOI-biosensor with ground electrodes located directly ontop of the microchip crystal (see Figure 2 (a)). Ten SOI-biosensors on a crystal increase the likelihood of the target molecules adsorbtion to the nanowire surface, thus improving the reliability of detection results.
An important component of effective detection with a biosensor is the availability of a receptor layer, as well as the possibility to select the type of receptors and their immobilization technique. Currently, cTnI are predominantly detected using monoclonal antibodies. An alternative approach is the application of synthetic nucleic acid sequences (aptamers). In terms of interaction specificity, aptamers are comparable to antibodies. They can be obtained using an in vitro evolutionary approach (SELEX) without cell lines. They can restore their activity after thermal denaturation and renaturation and exhibit high stability under tough operating conditions. Moreover, aptamers can be chemically synthesized and modified [31, 42], which makes them more cost-effective in terms of time and material costs. Aptamers can also bind to target molecules in solutions with high ionic strength, which allows the detection of proteins in undiluted biological samples such as serum, blood, etc. [23, 42]. In this study, a highly specific anti-troponin I DNA aptamer (TnAp12t2) was developed and used as a receptor; the dissociation constant (KD) of the “troponin I + anti-troponin I DNA aptamer” complex amounted to 6×10–9 mol/L. The aptamer was immobilized by physical adsorption, which allowed preserving its spatial configuration and reactivity with a simultaneous reduction of the time to prepare the biosensor for operation.
The authors of this study demonstrated the label-free real time detection of cardiac troponin I in the clinical concentration range of 10–11–10–9 mol/L using the developed design of SOI-biosensor. It was established that detection of a sample using SOI-biosensor took approximately 200 s. One should note that high concentrations of target molecules can lead to biosensor cut-off mode and incorrect results (point (5) in Figure 4). Detection of the target molecules in low concentrations is complicated by the possibility of their adsorption on the nanowire surface of SOI-biosensor. For example, adding the aptamer solution on the crystal surface at point (1) (see Figure 4) did not change the Ids value of SOI-biosensors No.2, 3, 5, and 6. We assume that here aptamer molecules were not adsorbed on the nanowire surface. Thus, the likelihood of the receptor or target molecules adsorption is a key factor in detection by SOI-FET biosensor [13, 32]. A theoretical study of the target molecules detection using a SOI-biosensor is discussed in an earlier publication [45].
Conclusion
The designed SOI-biosensor provides for real time label-free detection of troponin I. Detection of a troponin I sample using the SOI-biosensor takes approximately 200 s. The biosensor sensitivity is ~10–11 mol/L of protein. It was found that the aptamer immobilization by physical adsorption allowed keeping its reactivity. In the experiment, a highly specific anti-troponin I DNA aptamer (TnAp12t2) showed a positive effective electric charge at the liquid sample–nanowire phase interface. The “troponin I + anti-troponin I DNA aptamer” complex had a negative effective electrical charge at the same interface.
Study funding. The study was conducted within the State Task Order of Rospotrebnadzor GZ-21/21 and the State Task Order under the budget subject “Study of molecular-genetic and molecular-biological mechanisms of common therapeutic diseases in Siberia to improve approaches to their early diagnosis and prevention”, 2024–2028 (FWNR-2024-0002), as well as within the the State Task Order of the Ministry of Science and Higher Education of the Russian Federation (project No.FWES-2022-0002).
Conflicts of interest. The authors have no conflicts of interest to declare.
References
- World Health Organization. The top 10 causes of death. December 9, 2020. URL: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death.
- Snezhitsky V.A., Yorsh I.R., Golyshko V.S., Litvinovich S.N. Infarkt miokarda: patofiziologicheskie mekhanizmy razvitiya, diagnosticheskaya strategiya i taktika lecheniya [Myocardial infarction: pathophysiological mechanisms of development, diagnostic strategy, and tactics of treatment]. Grodno: Grodnenskiy gosudarstvennyy meditsinskiy universitet; 2015; 328 p.
- Pan T.M., Wang C.W., Weng W.C., Lai C.C., Lu Y.Y., Wang C.Y., Hsieh I.C., Wen M.S. Rapid and label-free detection of the troponin in human serum by a TiN-based extended-gate field-effect transistor biosensor. Biosens Bioelectron 2022; 201: 113977, https://doi.org/10.1016/j.bios.2022.113977.
- Ojha N., Dhamoon A.S. Myocardial infarction. Treasure Island (FL): StatPearls Publishing; 2022. URL: https://europepmc.org/article/nbk/nbk537076#_article-25460_s13_.
- Gerhardt W., Nordin G., Ljungdahl L. Can troponin T replace CK MBmass as “gold standard” for acute myocardial infarction (“AMI”)? Scand J Clin Lab Invest Suppl 1999; 230: 83–89, https://doi.org/10.1080/00365519909168331.
- Morrow D.A., Cannon C.P., Jesse R.L., Newby L.K., Ravkilde J., Storrow A.B., Wu A.H.B., Christenson R.H., Apple F.S., Francis G., Tang W.; National Academy of Clinical Biochemistry. National Academy of Clinical Biochemistry Laboratory Medicine Practice Guidelines: clinical characteristics and utilization of biochemical markers in acute coronary syndromes. Clin Chem 2007; 115(13): e356–e375, https://doi.org/10.1161/circulationaha.107.182882.
- Daubert M.A., Jeremias A. The utility of troponin measurement to detect myocardial infarction: review of the current findings. Vasc Health Risk Manag 2010; 6: 691–699, https://doi.org/10.2147/vhrm.s5306.
- Dhara K., Mahapatra D.R. Review on electrochemical sensing strategies for C-reactive protein and cardiac troponin I detection. Microchem J 2020; 156: 104857, https://doi.org/10.1016/j.microc.2020.104857.
- Kong T., Su R., Zhang B., Zhang Q., Cheng G. CMOS-compatible, label-free silicon-nanowire biosensors to detect cardiac troponin I for acute myocardial infarction diagnosis. Biosens Bioelectron 2012; 34(1): 267–272, https://doi.org/10.1016/j.bios.2012.02.019.
- Sharma S., Jackson P.G., Makan J. Cardiac troponins. J Clin Pathol 2004; 57(10): 1025–1026, https://doi.org/10.1136/jcp.2003.015420.
- Oliveira D.C.d.B., Costa F.H.M., da Silva J.A.F. The integration of field effect transistors to microfluidic devices. Micromachines (Basel) 2023; 14(4): 791, https://doi.org/10.3390/mi14040791.
- George Kerry R., Ukhurebor K.E., Kumari S., Maurya G.K., Patra S., Panigrahi B., Majhi S., Rout J.R., Rodriguez-Torres M.d.P., Das G., Shin H.S., Patra J.K. A comprehensive review on the applications of nano-biosensor-based approaches for non-communicable and communicable disease detection. Biomater Sci 2021; 9(10): 3576–3602, https://doi.org/10.1039/d0bm02164d.
- Tran D.P., Pham T.T.T., Wolfrum B., Offenhäusser A., Thierry B. CMOS-compatible silicon nanowire field-effect transistor biosensor: technology development toward commercialization. Materials (Basel) 2018; 11(5): 785, https://doi.org/10.3390/ma11050785.
- Kim K., Park C., Kwon D., Kim D., Meyyappan M., Jeon S., Lee J.S. Silicon nanowire biosensors for detection of cardiac troponin I (cTnI) with high sensitivity. Biosens Bioelectron 2016; 77: 695–701, https://doi.org/10.1016/j.bios.2015.10.008.
- De Moraes A.C.M., Kubota L.T. Recent trends in field-effect transistors-based immunosensors. Chemosensors 2016; 4(4): 20, https://doi.org/10.3390/chemosensors4040020.
- Generalov V.M., Naumova O.V., Fomin B.I., P’yankov S.A., Khlistun I.V., Safatov A.S., Zaitsev B.N., Zaitseva E.G., Aseev A.L. Detection of Ebola virus VP40 protein using a nanowire SOI biosensor. Optoelectron Instrum Data Process 2019; 55: 618–622, https://doi.org/10.3103/s875669901906013x.
- Patolsky F., Zheng G., Hayden O., Lakadamyali M., Zhuang X., Lieber C.M. Electrical detection of single viruses. Proc Natl Acad Sci U S A 2004; 101(39): 14017–14022, https://doi.org/10.1073/pnas.0406159101.
- Panahi A., Sadighbayan D., Forouhi S., Ghafar-Zadeh E. Recent advances of field-effect transistor technology for infectious diseases. Biosensor (Basel) 2021; 11(4): 103, https://doi.org/10.3390/bios11040103.
- Wadhera T., Kakkar D., Wadhwa G., Raj B. Recent advances and progress in development of the field effect transistor biosensor: a review. J Electron Mater 2019; 48: 7635–7646, https://doi.org/10.1007/s11664-019-07705-6.
- Sadighbayan D., Hasanzadeh M., Ghafar-Zadeh E. Biosensing based on field-effect transistors (FET): recent progress and challenges. Trends Analyt Chem 2020; 133: 116067, https://doi.org/10.1016/j.trac.2020.116067.
- Cetin Y., Aydinlik S., Gungor A., Kan T., Avsar T., Durdagi S. Review on in silico methods, high-throughput screening techniques, and cell culture based in vitro assays for SARS-CoV-2. Curr Med Chem 2020; 29(38): 5925–5948, https://doi.org/10.2174/0929867329666220627121416.
- Chiang P.L., Chou T.C., Wu T.H., Li C.C., Liao C.D., Lin J.Y., Tsai M.H., Tsai C.C., Sun C.J., Wang C.H., Fang J.M., Chen Y. T. Nanowire transistor-based ultrasensitive virus detection with reversible surface functionalization. Chem Asian J 2012; 7(9): 2073–2079, https://doi.org/10.1002/asia.201200222.
- Thriveni G., Ghosh K. Advancement and challenges of biosensing using field effect transistors. Biosensors (Basel) 2022; 12(8): 647, https://doi.org/10.3390/bios12080647.
- Bulgakova A., Berdyugin A., Naumova O., Fomin B., Pyshnyi D., Chubarov A., Dmitrienko E., Lomzov A. Solution pH effect on drain-gate characteristics of SOI FET biosensor. Electronics 2023; 12(3): 777, https://doi.org/10.3390/electronics12030777.
- Vance S.A., Sandros M.G. Zeptomole detection of C-reactive protein in serum by a nanoparticle amplified surface plasmon resonance imaging aptasensor. Sci Rep 2014; 4: 5129, https://doi.org/10.1038/srep05129.
- Yang X., Wang Y., Wang K., Wang Q., Wang P., Lin M., Chena N., Tan Y. DNA aptamer-based surface plasmon resonance sensing of human C-reactive protein. RSC Adv 2014; 4(58): 30934–30937, https://doi.org/10.1039/c4ra05011h.
- Lin M.C., Nawarak J., Chen T.Y., Tsai H.Y., Hsieh J.F., Sinchaikul S., Chen S.T. Rapid detection of natriuretic peptides by a microfluidic LabChip analyzer with DNA aptamers: application of natriuretic peptide detection. Biomicrofluidics 2009; 3(3): 34101, https://doi.org/10.1063/1.3194283.
- Pur M.R.K., Hosseini M., Faridbod F., Ganjali M.R. Highly sensitive label-free electrochemiluminescence aptasensor for early detection of myoglobin, a biomarker for myocardial infarction. Microchim Acta 2017; 184: 3529–3537, https://doi.org/10.1007/s00604-017-2385-y.
- Jo H., Her J., Lee H., Shim Y.B., Ban C. Highly sensitive amperometric detection of cardiac troponin I using sandwich aptamers and screen-printed carbon electrodes. Talanta 2017; 165: 442–448, https://doi.org/10.1016/j.talanta.2016.12.091.
- Negahdary M., Behjati-Ardakani M., Sattarahmady N., Yadegari H., Heli H. Electrochemical aptasensing of human cardiac troponin I based on an array of gold nanodumbbells — applied to early detection of myocardial infarction. Sens Actuators B Chem 2017; 252: 62–71, https://doi.org/10.1016/j.snb.2017.05.149.
- Chandola C., Kalme S., Casteleijn M.G., Urtti A., Neerathilingam M. Application of aptamers in diagnostics, drug-delivery and imaging. J Biosci 2016; 41(3): 535–561, https://doi.org/10.1007/s12038-016-9632-y.
- Squires T.M., Messinger R.J., Manalis S.R. Making it stick: convection, reaction and diffusion in surface-based biosensors. Nat Biotechnol 2008; 26(4): 417–426, https://doi.org/10.1038/nbt1388.
- Krasitskaya V.V., Goncharova N.S., Biriukov V.V., Bashmakova E.E., Kabilov M.R., Baykov I.K., Sokolov A.E., Frank L.A. The Ca2+-regulated photoprotein obelin as a tool for SELEX monitoring and DNA aptamer affinity evaluation. Photochem Photobiol 2020; 96(5): 1041–1046, https://doi.org/10.1111/php.13274.
- Stern E., Wagner R., Sigworth F.J., Breaker R., Fahmy T.M., Reed M.A. Importance of the Debye screening length on nanowire field effect transistor sensors. Nano Lett 2007; 7(11): 3405–3409, https://doi.org/10.1021/nl071792z.
- Generalov V.M., Naumova O.V., P’yankov S.A., Kolosova I.V., Safatov A.S., Zaytsev B.N., Zaytseva E.G., Buryak G.A., Cheremiskina A.A., Filatova N.A., Aseev A.L. Indication of the vaccinia virus by a nanowire silicon-on-insulator biosensor. Avtometria 2021; 57(1): 42–49, https://doi.org/10.15372/aut20210105.
- Ocaña C., del Valle M. A comparison of four protocols for the immobilization of an aptamer on graphite composite electrodes. Microchim Acta 2014; 181: 355–363, https://doi.org/10.1007/s00604-013-1126-0.
- Tan S.Y., Acquah C., Tan S.Y., Ongkudon C.M., Danquah M.K. Characterisation of charge distribution and stability of aptamer-thrombin binding interaction. Process Biochem 2017; 60: 42–51, https://doi.org/10.1016/j.procbio.2017.06.003.
- Kantor C.R., Schimmel P.R. Biophysical chemistry: part I: the conformation of biological macromolecules. 1st edition. W.H. Freeman and Company; 1980.
- Ravindranathan S., Butcher S.E., Feigon J. Adenine protonation in domain B of the hairpin ribozyme. Biochemistry 2000; 39(51): 16026–16032, https://doi.org/10.1021/bi001976r.
- Kochetkov N.K., Budovskiy E.P., Sverdlov E.D., Simukova N.K., Turchinskiy M.F., Shibaev V.N. Organicheskaya khimiya nukleinovykh kislot [Organic chemistry of nucleic acids]. Moscow: Khimiya; 1970.
- Shabarova Z.A., Bogdanov A.A. Khimiya nukleinovykh kislot i ikh komponentov [Chemistry of nucleic acids and their components]. Moscow: Khimiya; 1978; p. 584.
- Farrow T., Laumier S., Sandall I., van Zalinge H. An aptamer-functionalized Schottky-field effect transistor for the detection of proteins. Biosensors (Basel) 2022; 12(5): 347, https://doi.org/10.3390/bios12050347.
- Filatov V.L., Katrukha A.G., Bulargina T.V., Gusev N.B. Troponin: structure, properties, and mechanism of functioning. Biochemistry (Mosc) 1999; 64: 969–985.
- Serdechnyy troponin I [Cardiac troponin I]. HyTest; 2019. URL: https://hytest.ru/sites/5cd13840ff4f702c0cbc4c8d/ assets/5da43ec3fd7fb419e85444c7/ Troponin_Booklet_2019.pdf.
- Generalov V., Cheremiskina A., Glukhov A., Grabezhova V., Kruchinina M., Safatov A. Investigation of limitations in the detection of antibody+ antigen complexes using the silicon-on-insulator field-effect transistor biosensor. Sensors (Basel) 2023; 23(17): 7490, https://doi.org/10.3390/s23177490.