The Sensitivity of Hela Kyoto Cell Line Transfected with Sensor HyPer2 to Cisplatin
The aim of the investigation is to compare by means of MTT assay cytotoxic effect of cisplatin on the cells of HeLa Kyoto line and HeLa Kyoto line containing genetically-encoded sensor of hydrogen peroxide HyPer2 (HeLa Kyoto–HyPer2 line), and using staining by trypan blue to identify the doses of cisplatin causing cell death at different exposure time.
Materials and Methods. A HeLa Kyoto cell line of human cervical carcinoma and HeLa Kyota line transfected with the cytoplasmic sensor of hydrogen peroxide (HeLa Kyoto–HyPer2) were used in the study. The analysis of cytotoxic and antiproliferative action of cisplatin in relation to the given cells was performed using MTT assay. Cell viability was determined after 24 h of incubation with the preparation at concentrations from 0 to 50 μmol/L, then within the period from 0 to 24 h with an interval of 2 h at concentration of IC50; and also after 2, 4, 6, 8 h at concentrations from 9.3 to 833.3 μmol/L a quantity of live and destructed cells was counted using staining by trypan blue.
Results. After cisplatin expose the dose-response curves for cell viability of Hela Kyoto and HeLa Kyoto–HyPer2 cell lines were built according to MTT assay data. It was established that concentration of IC50 corresponding to the dose causing a loss of viability of 50% of cells is 1.3 times lower for HeLa Kyoto–HyPer2 compared to HeLa Kyoto. The results of staining by a vital agent trypan blue showed that inhibiting effects of cisplatin in concentration of IC50 by 24 h are mainly linked with the delay of cell division but not with their death. At concentrations up to 52 μmol/L damage of the membranes does not occur during 8 h, and at superhigh concentrations — 416.7 μmol/L — the damage is possible already 4 h after the exposure.
Conclusion. Comparison of sensibility of the two cell lines to the effect of cisplatin showed that transfection of the cells with the fluorescent protein results in the increase of the sensitivity to cisplatin. When HeLa Kyoto–HyPer2 cells are exposed to the preparation at concentration of IC50 during 24 h, inhibition of cell division is observed; higher concentrations of the preparation cause increase of the number of dead cells and diminish the terms of their destruction.
Cisplatin is a chemotherapeutic preparation, applied in the clinical practice for the treatment of a number of malignant tumors. It causes DNA damage [1], and also induces production of reactive oxygen species (ROS), initiating tumor cell death [2, 3]. At present, genetically encoded sensors based on fluorescent proteins became widely used in order to assess participation of definite forms of ROS in cell reaction to various external effects including therapeutic ones [4–9]. Advantages of these sensors are high specificity and the suitability for long-term dynamic studies. Nevertheless, when investigating cytotoxic effects of medications in relation to transfected cell lines, it is important to take into consideration the possibility of foreign proteins, present in the cells, to affect their sensitivity to the preparations used [10, 11]. Thus, it is necessary to make comparative evaluation of the cytotoxic effect of chemotherapeutic agents on transfected and original cell lines.
Besides, cisplatin demonstrates a number of dose-dependent effects against cells, e.g. influence on the ways of cell destruction, signal cascades, glycolytic activity, etc. [12, 13]. Therefore an important aspect of studying mechanisms of cytotoxic effects is a selection of optimal working doses of the preparation.
The aim of the investigation is to compare by means of MTT assay cytotoxic effect of cisplatin on the cells of HeLa Kyoto line and HeLa Kyoto line containing genetically-encoded sensor of hydrogen peroxide HyPer2 (HeLa Kyoto–HyPer2 line), and identify the doses of cisplatin causing cell death at different exposure time by trypan blue staining.
Materials and Methods. Two cell lines were used in the work: human cervical carcinoma line HeLa Kyoto and the same cell line transfected by genetically encoded cytoplasmatic sensor HyPer2, designated for dynamic investigation of intracellular hydrogen peroxide level (HeLa Kyoto–HyPer2) [14]. The cell lines were provided by M.M. Shemyakin and Y.A. Ovchinnikov Institute of Bioorganic Chemistry (Russia). The analysis of cytotoxic action of cisplatin in relation to the cells was carried out by MTT assay, based on the ability of mitochondrial dehydrogenases in the viable cells to convert water-soluble 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolum bromide (MTT) to formazan, which crystallizes inside the cell. Measurement of formazan concentration in the solution after the interaction with dimethylsulfoxide (DMSO) enables to calculate a number of viable cells, and in cytotoxic studies — to estimate a specific cell death, induced by one or the other agent [15].
Cells were seeded in 96-well plate in the number of 3000 cells per 1 well in 200 μl of Dulbecco’s Modified Eagle Medium (DMEM) (PanEcho, Russia), containing 2 mmol/L of glutamine and 10% FBS (Hyclone, USA), then were put into CO2-incubator for 24 h (37.0°C, 5% CO2). In 24 h the initial medium was replaced by DMEM with glutamine and 10% FBS, containing cisplatin. Cisplatin concentrations varied from 0 to 50 μmol/L. In 24 h the medium was changed for the medium with MTT (0.5 mg/ml), 200 μl per well. The plate was placed into CO2-incubator for 4 h, after which the medium with MTT was removed, and 100 μl of DMSO solution was added to each well and a resulting formazan was extracted during 40 min under constant stirring. Optic density of the obtained formazan solution in DMSO was measured on the plate spectrophotometer Synergy MX (BioTek, USA) at 570 nm wavelength. Intensity of color in the wells containing cells not treated by cisplatin was taken as 100% viability. On the graph (Fig. 1), showing dependence of the number of viable cells on cisplatin concentration, mean values of the data of 6 experiments for HeLa Kyoto–HyPer2 and 3 experiments for HeLa Kyoto, each of which was made in 3-fold repetition, and standard errors of mean are presented. Cytotoxicity curve building, determination of the dose causing viability loss of 50% of cells (IC50), identification of 95% confidence interval, nonlinear regression, and comparison of the curves by IC50 and the curve slope were performed by the program GraphPad Prism 6.01. A statistical significance level of differences between IC50 values, obtained from initial and transfected HeLa Kyoto cells treated by cisplatin, was estimated according to Fisher criterion. Values p<0.05 were recognized statistically significant.
 Fig. 1. Inhibiting effect of various concentrations of cisplatin on HeLa Kyoto and HeLa Kyoto–HyPer 2 lines (MTT assay) Fig. 1. Inhibiting effect of various concentrations of cisplatin on HeLa Kyoto and HeLa Kyoto–HyPer 2 lines (MTT assay)
|
Using the method of trypan blue staining [16] a percent content of the killed and survive cells after incubation with cisplatin in different concentrations was calculated for HeLa Kyoto–HyPer2 line. This method is based on the ability of the dye to penetrate inside the cell via the damaged membranes, leaving alive cells unstained.
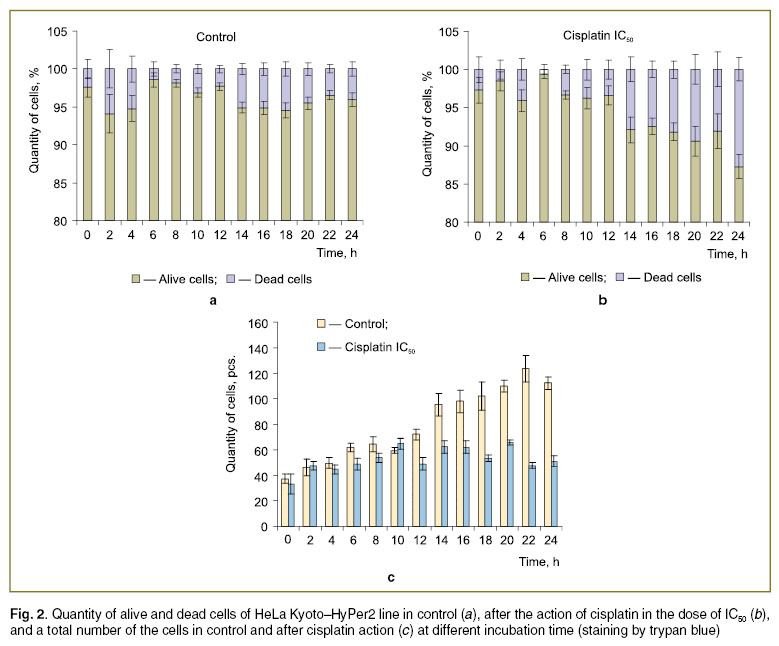
The day before the experiment the cells were seeded in the 12-well plate in the number of 150 thousand per well. Culturing was done in the DMEM with 2 mmol/L of glutamine and 10% of FBS. The time of exposure with cisplatin in the concentration corresponding to IC50 (8.3 μmol/L) amounted from 0 to 24 h with an interval of 2 h. The exposure time with the preparation at concentrations 13; 26; 52; 104; 208.3; 416.7; 833.3 μmol/L was 2, 4, 6, 8, 24 h. In control, DMEM medium with 2 mmol/L glutamine with 10% FBS without cisplatin was added to the cells. The time of exposure was 0–24 h with 2 h interval. After the exposure the medium from the wells was transferred to the microcetrifuge tubes. The cells were removed by the solution of trypsin-Versene (1:1) in the amount of 750 μl, and exposed in CO2-incubator for 5–7 min. Then all the content of the well was transferred into the appropriate centrifuge tube. The cells were centrifuged in a Microspin FV-2400 mini-centrifuge/vortex (BioSan, Latvia, 2400 rev/min) for 5 min. After supernatant removal a solution of PBS (700 μl) was added. Trypan blue (0.4% solution) was used for staining. Cells were counted in a Goryayev chamber, and the results were presented as a percentage of the alive and destructed cells of their total number. Mean arithmetic values, obtained in two-fold repetition in 3 wells, and their standard errors are given on the diagrams.
Results. According to MTT assay data, a curve of viability of HeLa Kyoto and Hela Kyoto–HyPer2 cells against concentration of cisplatin in the medium (See Fig. 1) was built. It was estimated that IC50 concentration corresponding to the dose causing the viability loss of 50% of the cells (with a 95% confidence interval) amounts to 8.3 μmol/L [7.307; 9.447] for HeLa Kyoto–HyPer2 cells, and 10.5 μmol/L [8.4; 13.9] — for HeLa Kyoto. Differences between the values IC50 for the studied cell lines appeared to be statistically significant (p=0.04).
Trypan blue staining (Fig. 2, 3) enabled evaluating dose-time dependence of the response of the HeLa Kyoto–HyPer2 line cells to cisplatin action. The number of dead cells after cisplatin expose in the IC50 concentration was found to increase in 14 h and continued to grow to 24 h (up to the end of the experiment, Fig. 2, b). By 24 h the number of the cells died after treatment increased, at the average, from 2.7±1.7 to 12.7±1.5% compared to the untreated control cells (Fig. 2, a, b). Notable, that the total number of the cells did not increase in the presence of cisplatin, i.e. their division did not take place, while the quantity of the control untreated cells increased, at the average, 2.3 times (Fig. 2, c). When higher doses of the preparation are used, decrease of the viable cell number from 95.5±0.2 to 40.3±6.0% was observed already in 4 h, and 80–90% of the cells were dead in 6 h (833.3 μmol/L, Fig. 3, c), in 8 h (416.7 μmol/L, Fig. 3, d), in 24 h (26 μmol/L, Fig. 3, e).
Discussion. Standard MTT assay and trypan blue staining allowed identifying cisplatin concentrations causing death of a certain quantity of HeLa Kyoto and HeLa Kyoto–HyPer2 cells.
Cytotoxicity curves of both cell lines demonstrate similar dynamics indicating their similar sensitivity to the preparation. As the knee of the curves is relatively short and their slope is slightly steeper than a standard one [17] (the curve slope for HeLa Kyoto: –1.876 [–2.583; –1.170], for HeLa Kyoto–HyPer2: –2.196 [–2.739; –1.652]), conclusion can be drawn on a relatively high sensitivity of the both lines to the preparation.
The value of IC50 obtained in our work corresponds the concentration presented in [18] for the HeLa line. Regression analysis data showed that IC50 value for transfected HeLa Kyoto cells was found to be 1.3 times less than for non-transfected ones (See Fig. 1). This is the evidence of a slightly higher sensitivity of HeLa Kyoto cells containing HyPer2 sensor to cisplatin which may be connected with the presence of the fluorescent protein in the cell. The sources of such increased sensitivity of the transfected cells may be various. Thus, there are data concerning the induction of the oxidation process caused by the introduction of the green fluorescent protein (GFP) into the cell, and on the respective elevation of the sensitivity of the transfected cell lines to the action of such chemotherapeutic agents as carboplatin, doxorubicin, etoposid, melphalan in comparison with the wild-type cells [10]. An increased sensitivity of the cells transfected by GFP or its analogs (yellow fluorescent protein or enhanced GPF) to antitumor preparations is closely linked with the production of ROS, singlet oxygen in particular [11]. In response to GFP transfection and an oxidative stress induced by it, the increase production of glutation is observed [10]. It may be suggested that the factor mentioned lie in the basis of resistance reduction of HeLa Kyoto–HyPer2 line compared to HeLa Kyoto.
Our investigation demonstrated that a 50% decrease in HeLa Kyoto–HyPer2 cell viability, found by MTT assay data, under the action of cisplatin in the concentration of IC50 in 24-h incubation. That is determined not so much due to cell death but rather due to the inhibition of cell division (See Fig. 2).
In order to identify cisplatin doses causing cell death in the period within 24 h, a number of incrementing preparation doses was used (from 13 μmol/L, which exceeds IC50 by 1.6 times, according to the MTT assay data, and corresponds approximately to the dose causing a viability loss of 80% of cells as the findings of the MTT assay showed, up to super-high doses). The increase of preparation concentration resulted in a more rapid cell death (See Fig. 3).
In [12] it was shown that for normal as well as for tumor cells cisplatin concentration of less than 30 μmol/L in the majority of cases induces apoptosis, concentrations of more than 300 μmol/L induces necrosis, and treatment by cisplatin in the concentration of 100 μmol/L causes both ways of cell destruction. The authors registered characteristic signs of necrosis and apoptosis development starting from 8 h of expose independently on the preparation concentration. Methods used in our investigation do not allow identifying exactly the way HeLa Kyoto–HyPer2 cells death after cisplatin exposure because trypan blue stains both necrotic and apoptotic cells [16]), but allow identification of dose-time parameters of the action.
The results of cell staining with trypan blue showed that cell membranes became damaged mainly after 8 h already at relatively low cisplatin concentrations — from 52 μmol/L. In 4 and 6 hours cells became stained only when exposed with cisplatin at concentrations over 416.7 μmol/L. Consequently, the processes of necrosis or late apoptosis, damaging the membranes, are likely to be triggered at a short time exposure only at high concentrations. Lower concentrations of the preparation are also able to cause necrosis and late apoptosis if exposure is long. At low concentrations, comparable with IC50, inhibition of cell division not leading to their death during 24 h is likely to occur. At higher concentrations — up to 52 μmol/L — the cell death may take place but it is connected with the processes of early apoptosis during the first 8 h of the exposure (such cells are not stained by trypan blue), after this time late apoptosis and necrosis may be observed. High concentrations of cisplatin are capable of causing cell death already at low concentrations.
The results obtained are believed to be rather important in following aspects. Firstly, differences in cell line sensitivity and dose-dependent effects of the exposure found in the course of the investigation should be taken into consideration when optimal working concentrations of the preparation are calculated. Secondly, the findings of the study are necessary for understanding the character of toxic preparation effect in relation to HeLa Kyoto–HyPer2 line, carrying genetically-encoded sensor, and its further application in the analysis of the hydrogen peroxide role in the mechanism of cell death, induced by cisplatin.
Conclusion. A comparative study of cisplatin effect in relation to HeLa Kyoto and HeLa Kyoto–HyPer2 cell lines has found out that transfection of the cells by a fluorescent protein causes statistically significant increase of sensitivity to the preparation. Concentration of cisplatin corresponding to IC50 acting during 24 h does not lead HeLa Kyoto–HyPer2 cells to death, but inhibit their division. At concentrations of cisplatin less than 52 μmol/L damage of membranes does not show itself during 8 h, but when concentrations are high — 416.7 μmol/L — damage is possible already after 4 h of exposure. Thus, it is the time interval from 0 to 8 h after addition of cisplatin in the dose of less than 52 μmol/L that is optimal for studying the processes of early apoptosis in the given line of tumor cells. These data should be taken into account when studying molecular mechanisms of damaging normal and tumor cells under the action of cisplatin using genetically-encoded sensors.
Study Funding and Competing Interest. This work was supported by grants from Russian Foundation for Basic Research (No.13-04-97165, No.13-04-40228-H), grants from the Ministry of Education and Science of the Russian Federation (No.11.G 34. 31.0017, No.14.Z50.31.0022). There is no topic specific conflict of interest related to the authors of this study.
Acknowledgements. The authors would like to thank Lukyanov S.A., D.Bio.Sc., and Belousov V.V., D.Bio.Sc., for providing cell lines, Balalayeva I.V., PhD, Zdobnova T.A., PhD, and Sokolova E.A. for their assistance in preparing and performing experiments, and also in analyzing the data.
References
- Johnson N.P., Butour J.-L., Villani G., Wimmer F.L., Defais M., Pierson V., Brabec V. Metal anti-tumor compounds: the mechanism of action of platinum complexes. Prog Clin Biochem Med 1989; 10: 1–24.
- Itoh T., Terazawa R., Kojima K., Nakane K., Deguchi T., Ando M., Tsukamasa Y., Ito M., Nozawa Y. Cisplatin induces production of reactive oxygen species via NADPH oxidase activation in human prostate cancer cells. Free Radic Res 2011; 45(9): 1033–1039, http://dx.doi.org/10.3109/10715762.2011.591391.
- Katsuda H., Yamashita M., Katsura H., Yu J., Waki Y., Nagata N., Sai Y., Miyamoto K. Protecting cisplatin-induced nephrotoxicity with cimetidine does not affect antitumor activity. Biol Pharm Bull 2010; 33(11): 1867–1871, http://dx.doi.org/10.1248/bpb.33.1867.
- Wang W., Fang H., Groom L., Cheng A., Zhang W., Liu J., Wang X., Li K., Han P., Zheng M., Yin J., Wang W., Mattson M.P., Kao J.P., Lakatta E.G., Sheu S.S., Ouyang K., Chen J., Dirksen R.T., Cheng H. Superoxide flashes in single mitochondria. Cell 2008; 134(2): 279–290, http://dx.doi.org/10.1016/j.cell.2008.06.017.
- Belousov V.V., Fradkov A.F., Lukyanov K.A., Staroverov D.B., Shakhbazov K.S., Terskikh A.V., Lukyanov S. Genetically encoded fluorescent indicator for intracellular hydrogen peroxide. Nat Methods 2006; 3(4): 281–286.
- Markvicheva K.N., Bogdanova E.A., Staroverov D.B., Lukyanov S., Belousov V.V. Imaging of intracellular hydrogen peroxide production with HyPer upon stimulation of HeLa cells with epidermal growth factor. Methods Mol Biol 2009; 476: 76–83.
- Pearce L.L., Gandley R.E., Han W., Wasserloos K., Stitt M., Kanai A.J., McLaughlin M.K., Pitt B.R., Levitan E.S. Role of metallothionein in nitric oxide signaling as revealed by a green fluorescent fusion protein. Proc Natl Acad Sci USA 2000; 97(1): 477–482, http://dx.doi.org/10.1073/pnas.97.1.477.
- Belova А.S., Mishina N.M., Orlova А.G., Sergeeva Е.А., Maslennikova А.V., Brilkina А.А., Shakhova N.М., Belousov V.V., Lukyanov S.А. The study of cisplatin effect on hydrogen peroxide and PH level in Hela Kyoto cell line using genetically-encoded sensors. Sovremennye tehnologii v medicine 2013; 5(4): 19–24.
- Belova A.S., Orlova A.G., Maslennikova A.V., Brilkina A.A., Balalaeva I.V., Antonova N.O., Mishina N.M., Shakhova N.M., Belousov V.V. The study of hydrogen peroxide level under cisplatin action using genetically encoded sensor HyPer. Proceedings of SPIE 2014; 8956: 895612, http://dx.doi.org/10.1117/12.2037737.
- Goto H., Yang B., Petersen D., Pepper K.A., Alfaro P.A., Kohn D.B., Reynolds C.P. Transduction of green fluorescent protein increased oxidative stress and enhanced sensitivity to cytotoxic drugs in neuroblastoma cell lines. Mol Cancer Ther 2003; 2(9): 911–917.
- Greenbaum L., Rothmann C., Lavie R., Malik Z. Green fluorescent protein photobleaching: a model for protein damage by endogenous and exogenous singlet oxygen. Biol Chem 2000; 381: 1251–1258, http://dx.doi.org/10.1515/BC.2000.153.
- Sancho-Martínez S.M., Piedrafita F.J., Cannata-Andía J.B., López-Novoa J.M., López-Hernández F.J. Necrotic concentrations of cisplatin activate the apoptotic machinery but inhibit effector caspases and interfere with the execution of apoptosis. Toxicol Sci 2011; 122(1): 73–85, http://dx.doi.org/10.1093/toxsci/kfr098.
- Schwerdt G., Freudinger R., Schuster C., Weber F., Thews O., Gekle M. Cisplatin-induced apoptosis is enhanced by hypoxia and by inhibition of mitochondria in renal collecting duct cells. Toxicol Sci 2005; 85(1): 735–742, http://dx.doi.org/10.1093/toxsci/kfi117.
- Markvicheva K.N., Bilan D.S., Mishina N.M., Gorokhovatsky A.Yu., Vinokurov L.M., Lukyanov S., Belousov V.V. A genetically encoded sensor for H2O2 with expanded dynamic range. Bioorganic & Medicinal Chemistry 2011; 19(3): 1079–1084, http://dx.doi.org/10.1016/j.bmc.2010.07.014.
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 1983; 65(1–2): 55–63, http://dx.doi.org/10.1016/0022-1759(83)90303-4.
- Louis K.S., Siegel A.C. Cell viability analysis using trypan blue: manual and automated methods. Methods Mol Biol 2011; 740: 7–12, http://dx.doi.org/ 10.1007/978-1-61779-108-6_2.
- Freshni R.Ya. Kul’tura zhivotnykh kletok [Animal cell culture]. Moscow: BINOM. Laboratoriya znaniy; 2010; 691 р.
- Komleva N.V., Kostyuk G.V., Parkhomenko I.I., Balalaeva I.V., Golubev V.A., Sen V.D., Terentev A.A. Comparative analysis of cytotoxicity and the effect of platinum (IV) complexes with aminonitroxyl radicals on the cell cycle. Vestnik Nizhegorodskogo universiteta im. N.I. Lobachevskogo 2011; 2(2): 82–89.