Fenton’s Reaction Induced Chemiluminescence Is Mathematical Modeling of the Process; Characteristics, Parameters and Application Conditions for Biomedical Studies
The aim of the investigation was to simulate mathematically the process of chemiluminescence induced by Fenton’s reaction; and reveal the characteristics, parameters and application conditions of Fenton’s reaction for biomedical studies.
Materials and Methods. We calculated chemiluminescent pulse resulting from Fenton’s reaction based on numerical solution of a set of 19 differential equations describing the process; the calculation being performed without any simplifying assumptions. 25 possible reactions in Fenton’s reagent (hydrogen peroxide and ferrous iron solution) and 7 reactions of chain oxidation of organic substance RH were studied. The system of equations was solved using a software package MathCad 14. Experimentally, luminescence pulse characteristics were measured by a biochemiluminometer BKhL-07 (Russia). We used distilled water; reagents: [Fe2+] concentration 10−4 and 10−3 mol/L, [H2O2] concentration 10−4 and 10−3 mol/L; biological substrates: albumin 50 g/L, blood plasma, erythrocytes and whole blood of intact Wistar male rats.
Results. We analyzed the use of ferrous iron and the shape of chemiluminescent pulses arising from Fenton’s reaction, yields of chemiluminescence (light sum) with hypothetical organic RH substance at different rate constant values of chain initiation, continuation and disconnection reactions in relation to RH substance concentration. The recording time was 30 s. Light sum (chemiluminescence intensity) in all cases was found to be maximum achieved under certain RH concentrations. Maximum position depends on the substance properties. Under certain conditions an oxidative chain reaction can proceed, hydroperoxide ROOH forms, with no luminescence occurring. The obtained radiation pulse shapes were experimentally confirmed. In studies with biological substrates and organic substances, the light sum index of chemiluminescence induced by Fenton’s reaction appeared to be stable if the concentrations of the components were as follows [Fe2+]=10−3 mol/L, [H2O2]=10−3 mol/L, pH=2.
Fenton’s reaction occurring when mixing solutions of transition metals, ferrous iron and hydrogen dioxide, in particular, was first reported in 1894. The reaction is of interest due to the fact that it generates the strongest oxidizer — hydroxyl radical OH•, able to oxidize any organic substance. One of the applications of Fenton’s reaction is the study of chemiluminescence of biological substrates. Chemiluminescence as a research method has been started to be widely used since high-sensitivity biochemiluminometers were developed. The study has considered the possibilities of chemiluminescence application including that occurring in Fenton’s reaction [1].
In biomedical studies using a biochemiluminometer, chemiluminescence intensity is recorded immediately after mixing all components (a bioassay, Fe2+ and H2O2 solutions). Measuring time is 30–60 s. Such variant of chemiluminescence study using Fenton’s reaction has been applied in many research works [2–5]. In particular, in the study [2] for analysis there was used total yield of recorded radiation S (light sum), maximum luminescence intensity Imax and slope ratio of trailing edge of radiation pulse tgα. These parameters are assigned certain sense, though the relationship of these characteristics of radiation pulse with chain oxidation reaction has not been analyzed in literature. The researchers [3] reported that Fenton’s reaction resulted in the formation of unstable tetroxide degrading with light quantum emission. By the term “tetroxide” must be meant a complex (Fe2+Fe23+)O42– (iron(II,III)oxide). This complex can be formed, since Fe2+ is a basic substance, and Fe3+ is a resultant. Iron(II,III)oxide is a stable mineral, named as magnetite, which makes no contribution to luminescence [6]. The formation of iron compounds with higher oxidation than Fe3+ in Fenton’s reaction is unknown. Therefore, the problem of luminescence mechanism requires more detailed consideration.
The study [7] reports of the program “Kinetics” developed for mathematic kinetics simulation of complex reaction systems. Antioxidant activity of substances has been studied using the program. In the model luminescence is supposed to occur when there is the interaction of two radicals LOO• (L — lipid). The number of equations can be solved using the program is limited, so for particular tasks one has to introduce simplifying assumptions.
Another work [8] represents a model of processes in Fenton’s reaction taking into account all possible reactions between the products, as well as the scheme of luminescence formation both in Fenton’s reaction itself, and in a chain reaction initiated by hydroxyl radicals. Some characteristics were experimentally verified during the investigation [8], and when studying pro-oxidant and anti-oxidant properties of ascorbic acid [9].
Currently there has been gained a lot of background material on kinetic constants of radical reactions between themselves and with particular substances. There have been studied in detail the characteristics of chain reactions initiated by hydroxyl radicals in organic substance tests. Therefore, it is possible to calculate accurately luminescent pulse characteristics and its relationship with oxidative chain reaction peculiarities.
The aim of the investigation was to simulate mathematically and calculate pulse characteristics of luminescence occurring just in Fenton’s reaction, and in the reaction with organic substance RH; and reveal the characteristics, parameters and application conditions of Fenton’s reaction for biomedical studies.
Materials and Methods. Luminescence in Fenton’s reaction was recorded using a biochemiluminometer BKhL-07 (Russia). Sensitivity of the device was ~200 photon/s. The device was calibrated against reference light source (luminescent uranium glass JS-19) before work starting [10]. The volume of the tray for reagents was 2 ml, diameter — 1 cm. The following reagents were used: FeSO4 solution at concentration 10–3 and 10–4 mol/L in acidic medium pH=2, hydrogen peroxide solution at concentration 10–3 and 10–4 mol/L. Acidic medium for iron solution was made by adding sulfuric acid due to the fact that ferrous iron is unstable in a neutral medium.
Test composition was the following: FeSO4 solution, 0.4 ml; solvent (distilled water, Hank’s solution), 0.4 ml; 0.1 ml of test substance or biological substrate under study (in control test — 0.1 ml of solvent); hydrogen peroxide, 0.2 ml. After adding hydrogen peroxide, the cell holder was prepared for operation position: a measuring camera was darkened, and the tray was placed opposite the photomultiplier tube (PMT) cathode. Radiation recording started automatically, no more than 1 s after the recording started. Recording time was 30 s. Background (PMT noise current) was subtracted automatically. We recorded time dependence of luminescence intensity. The device showed maximum signal amplitude — Imax, light sum — S throughout the measuring period, and slope ratio of the trailing edge of radiation pulse — tgα. The luminescence value directly measured by BKhL-07 is voltage intensity, since S and Imax were measured in mV (relative units). Albumin solution, 50 g/L, was used as the test substance; and Wistar male rat heparinized whole blood, blood plasma, erythrocytes — as biological substrates. Blood plasma and erythrocytes were taken after decapitation and centrifuged for 10 min at 3000 rpm. Experimentally, within 30 s we measured chemiluminescence light sum of a blank sample S0 and used in the survey albumin, erythrocytes, blood plasma and whole blood S at various concentrations (dilutions) in 10–15 repeats. We started measurements with light sum S recording at initial concentration followed by serial dilution by Hank’s solution by 10 times obtaining light sums at the concentrations 1, 10–1, 10–2, … 10–10. For spectral band assessment we used: blue (band pass 410–590 nm) and red (band pass 590–750 nm) light filters.
The process initiated by Fenton’s reaction was calculated using numeric calculations of chemical kinetic equations. We took into consideration carbon dioxide uptake by distilled water from air, and ambient radiation background [11]. We wrote 19 differential equations describing the accumulation and discharge of the following substances and active particles: Fe2+, Fe3+, H2O2, HO2–, O2, HO2•, OH•, O2•–, 1O2 (singlet oxygen), 21O2 (singlet oxygen dimer), HCO3–, CO3•–, RH (conditional test substance), R•, ROO•, ROOH, ROOR, RO•, R-R [8]. We calculated both the particles participating in Fenton’s blank reaction, and the particles resulting from a chain process initiated by Fenton’s reaction in a biological substrate. The calculation was performed for reaction time 60 and 30 s. The set of 19 differential equations was solved using MathCad 14. We additionally checked the solution for stability, since the solution of a set of equations (even two) is an unstable (ill-defined) problem. It means that basically there can be conditions, when infinitestimal change of initial data results in infinitely great changes of the solution.
The solution of the set of equations was the relation of the dependence of the concentration of each substance and active particle on time since the reaction start. Modeling problem consisted in the investigation of chemiluminescence properties relying on known characteristics of reactions proceeding in the system. Variation in a wide range of initial data and concentrations of reagents enables to reveal regularities, which can be observed in an experiment, and design experimental set up. The theory of chain reactions has been studied for nearly a hundred of years, while the characteristics of all reactions are well studied. Therefore, the assigned task can be solved accurately.
Results and Discussion
Fenton’s reaction behavior characteristics. An initial stage is described by the following reaction:

The kinetic constant of the reaction is k1=56 L(mol·s)–1. To obtain stable results, the principal condition of the reaction behavior is an acidic medium, pH=2. In a neutral medium iron is rapidly oxidized by atmospheric oxygen (Fe2+→Fe3+) and the concentration of ferrous iron solution initially used decreases. In addition, with pH increase kinetic constant of Fenton’s reaction decreases, i.e. the reaction characteristics alter. The kinetic constant k1=56 L(mol·s)–1 used in the calculation [8] has been reported in reference literature for pH=2. All stages of reaction continuation have been considered in the research paper [8]. When preparing pH it is sulfuric acid that is to be added, since chlorohydric acid has chloride ions, which are oxidized by hydroxyl radical.
Fenton’s reaction is used as the source of hydroxyl radicals OH•. Secondary products of the reaction are radicals HO2•, O2•– and singlet oxygen 1O2. The formation of ion-radical O2•– provides a counter reaction:

The reaction of O2•– with hydrogen ion results in singlet oxygen formation:

Singlet oxygen is a basic luminescent agent, which is the reaction resultant and can be recorded by PMT. A complete description of luminescence formation in Fenton’s reaction is presented in the scheme including 25 reactions [8]: reactions with dicarbonates accumulated in water as a result of carbon dioxide dissolved in the air, as well as the formation of active particles when exposed to external radiation background [11]. Many by-products resulting from the reaction (1) and initiated by hydroxyl radicals can be formed at excited states, though the energy of such states corresponds to an ultraviolet band. The radiation spectrum of such states has been given in the study [12]. Spectrum maximum is at wavelength ~300 nm, there is practically no radiation over 380 nm. Therefore, the photons deactivating/de-energizing these states cannot be recorded by PMT, its spectral range of sensitivity starting from 400 nm.
Decomposition of singlet oxygen itself is accompanied by photon (wavelength of 1260 nm) emission; the photons are not recorded by PMT. PMT records chemiluminescence of the process of singlet oxygen dimer formation and decomposition:

Fundamental wavelength of the resultant radiation is λ=630 nm. There are also less intensive lines of 480, 535 and 580 nm associated with the transitions from different states of singlet oxygen. Luminescence intensity of singlet oxygen dimer in a red spectral band is low, for this reason in the survey [12] this radiation was not recorded, since the maximum of singlet oxygen dimer emission is ~300 nm. In another study [13] the authors used luminol to intensify light output, though the sensitivity of modern luminometers is enough to record singlet oxygen dimer alone.
An experimental study of luminescence radiation spectral band revealed that radiation yield did not exceed 5% of the initial yield (with no filter) when there was a blue light filter; a red light filter reduced radiation yield at least by 10%. The findings have confirmed that chemiluminescence radiation induced by Fenton’s reaction is the spectral area corresponding to the emission lines of singlet oxygen dimer.
Water quality influences luminescence yield. First, active particles are formed in water under external radiation background [11]. Concentration setting time of such particles is ~30 min. Secondly, carbon dioxide taken up by water also has an effect on luminescence enhancing it. The amount of carbon dioxide absorbed is increasing if distilled water holding time in air increases. Therefore, to obtain stable results, one should use only fresh water though kept after distillation at least 30 min.
The recording of chemiluminescence of biological substrates has some peculiarities. In standard spectroscopy a recorded signal forms a lighting flash far exceeding the background in amplitude. A signal is isolated from the background by means of amplitude discrimination. In a biochemical reaction one photon is emitted, which knocks out one electron on PMT cathode. This electron is not different from those emitted by a cathode as a result of background thermal noises. The background effect can be distinguished only by the changed number of electrons emitted by a cathode. The effect of biological substrate chemiluminescence can be considered significant only if it exceeds the fluctuation of PMT noises. For this reason, serious demands are placed on PMT: noise should be minimal and stable. The background is subtracted automatically on BKhL-07. The measurements showed [8] that root mean square fluctuations of biochemiluminometer’s (BKhL-07) noise were from 200 to 500 pulses per second depending on the device state that corresponds to output voltage (chemiluminescence intensity) I~1 mV. The chemiluminescence effect of the test sample can be recorded only provided that radiation flotation on PMT cathode exceeds ~200 s–1 (~0.5 mV).
The selection of concentration for reagents. During Fenton’s reaction Fe2+ is expended, and if its concentration drops intensively, the reaction practically stops, therefore, Fe2+ concentration determines the reaction duration. If an initial concentration is [Fe2+]=10–3 mol/L, 60 s later Fe2+ concentration reduces by 10 times [14] and the reaction practically ceases. The period of time within which chemiluminescence can be recorded is determined by the characteristics of a measuring appliance. For BKhL-07 the chosen recording time was 30 s. Over 30 s Fe2+ concentration decreases about thrice, the reaction still persists, that is why concentration [Fe2+]=10–3 mol/L can be taken as an operating concentration for experimental conditions under consideration.
To assess Fenton’s reaction time one can use an approximate relationship [14]:

where k1=56 L(mol·s)–1 is the reaction rate constant (1). Provided that concentration is [Fe2+]≥[H2O2], accurate reaction time calculation is the following: τ~6 s if [Fe2+]=10–2 mol/L; τ~60 s if [Fe2+]=10–3 mol/L; τ~600 s if [Fe2+]=10–4 mol/L [14]. If the concentration is [Fe2+]<[H2O2], then the counter reaction (2) of Fe2+ reduction by a radical ion O2•– makes the great contribution, and the reaction rate will depend on peroxide concentration. Since hydrogen dioxide can be expended not only in the reaction (1), but also in reactions with analyzed organic substances, then the results can be unstable. Therefore, it is better not to use the mode [Fe2+]<[H2O2].
Hydrogen dioxide concentration in Fenton’s reaction determines chemiluminescence intensity, luminescence grows with concentration increase [8, 14]. However, the time of radiation burst and pulse-leading edge decreases. If [H2O2]=10–1 mol/L, the bulk of radiation is released during the period less than 0.1 s [14]. Transient processes associated with placing a tray with a test sample into a measuring position take at least a few tenths of a second, therefore, if [H2O2]=10–1 mol/L, only a “tail” of radiation (its small part) is recorded. To record almost the whole luminescence of Fenton’s reaction within 30–60 s, the peroxide concentration should be [H2O2]≤[Fe2+]. The survey [8] has calculated and proved experimentally the optimal concentration of hydrogen dioxide for distilled water to be [H2O2]=10–4 mol/L.
When Fenton’s reaction proceeds in a solution containing biological and organic substrates, the situation is different. Hydrogen peroxide will interact primarily with the organic substance under analysis, since the iron reaction rate constant is low, and rate constants of other hydrogen peroxide reactions can appear to be higher [15, 16]. Thus, hydrogen peroxide will be expended mainly in other reactions (not with ferrous iron) — in this case one should choose its higher concentration. Hydrogen peroxide concentration also depends on design features of a luminometer used. Hydrogen concentration [H2O2]~10–3 mol/L can be recommended for BKhL-07 with a concentration of organic substance specific to blood.
Chemiluminescence pulse form can help assess if the concentration of reagents has been decided on correctly. The form of experimental pulses at different concentrations [Fe2+] and [H2O2] (Fig. 1) shows that at the concentrations [Fe2+]=10–3 mol/L and [H2O2]=10–4 mol/L (Fig. 1, а) luminescence is low, and it finishes rapidly (2 s later). If the concentration is [Fe2+]=10–4 mol/L and [H2O2]=10–3 mol/L (Fig. 1, b), luminescence is also low, however, it does not finish over 30 s, since reduced [Fe2+] results in reaction time increase — see the relationship (5). If the concentration of the reagents we selected as working ones was [Fe2+]=[H2O2]=10–3 mol/L (Fig. 1, c), the luminescence almost four times more intensive, and the reaction is certain to proceed for 30 s. As was mentioned above, with the increase of reagent concentration the duration of pulse and its leading edge decreases. In addition, the considerable part of information (light sum) is lost, and the results can be unreliable. That is why in this study we did not measure chemiluminescence induced by Fenton’s reaction at the concentrations of reagents of over 10–3 mol/L.
Thus, the concentrations of over 10–3 mol/L of both reagents: [Fe2+] and [H2O2] cannot be recommended for investigations.
Calculation of luminescence induced in Fenton’s reaction with a biological test. To assess the substance oxidation power, Fenton reagent is added to the substance, and the resultant chemiluminescence (light sum S) yield is measured. Let us denote light sum recorded in Fenton’s reaction without a test sample as S0. Light sum S with a biological sample can increase, decrease or remain unchanged (in relation to S0) depending on the properties of a biological substrate. Hydroxyl radicals originated in Fenton’s reaction interact with organic substances of a sample initiating a chain oxidation reaction. Let us consider how the characteristics of an organic substance the biological sample contains, and the properties of resultant by-products influence chemiluminescence intensity.
A typical chain oxidation reaction of an organic substance by RH hydroxyl radicals can be represented as the sequence of the following reactions (k1–k10 — reaction mean rate constants (6)–(15)).
Initiation:

Chain continuation:

Chain disconnection, singlet oxygen formation:

Singlet oxygen reactions:
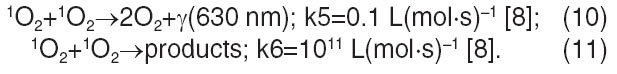
Formation of low-active products:

Some substances can be oxidized according to the mechanism, which has its peculiarities at the stages of a chain continuation, in particular, regarding ascorbic acid oxidation [9]. However, most organic substances oxidize according to the mechanism shown below.
All reactions from (6) to (15) are the second order reactions. Such reaction rate equals to the product of reagent concentrations raised to the power of their stoichiometric coefficients multiplied by a rate constant. All stoichiometric coefficients in the cases analyzed equal to 1. By the example of ROO• formation reaction rate let us write the following equation. Radicals ROO• form in the reaction (7) and are expended in the reactions (8) and (9):

As has been stated in the present study and was shown in the work [8], chemiluminescence in Fenton’s reaction is due to singlet oxygen dimer decomposition. Singlet oxygen is formd both in Fenton’s solution (a blank test) itself — reaction (3), and also when an organic substance RH is oxidized in reaction (9); singlet oxygen formation rate increasing compared to the rate of blank Fenton’s solution without a biological sample. Chemiluminescence occurs when singlet oxygen dimer decomposes. When calculating, singlet oxygen dimer formation rate was taken for chemiluminescence intensity, since singlet oxygen decomposes almost immediately:

The first stage of the reaction is initiation with a free radical R• forming — reaction (6). Rate constant of this reaction for different substances varies in a wide range and can reach k1=1010 L(mol·s)–1. With oxygen present, a radical ROO• is formed — reaction (7). If oxygen is absent, chain oxidation is impossible. The main stage of chain continuation is reaction (8), during which a molecule of the basic substance is expended, and radical R• is formed. This reaction is slow, its rate constant is in the range of 1–60 L(mol·s)–1, and it is a rate-limiting stage, which determines the chain oxidation rate. The reaction results in hydroperoxide formation, hydroperoxide being considered as the sign of chain oxidation. Chain disconnection when radicals ROO• interacting results in singlet oxygen 1O2 formation — reaction (9). Thus, the formation of radicals ROO• and their recombination with singlet oxygen formation enhances chemiluminescence intensity in Fenton’s reaction when analyzing biological samples. However, it is possible when reaction rate constant (8) is anomalously high, and luminescence will decrease in the process of substrate test. This case will be considered below.
The probability of singlet oxygen dimer formation is questionable, reaction rate constant (10) is k5=0.1 L(mol·s)–1, that is far less than the reaction rate constant (11). However, such reaction rate will be enough to record luminescence by a high-sensitive biochemiluminometer. Hydroperoxide can interact with ferrous iron — reaction (12), but rate constant of this reaction is low, therefore, it plays no critical role. Another reaction channel of chain disconnection is the radicals R• and RO• interaction — reactions (13), (14), (15). In these reactions low-active compounds are formed having no effect on luminescence. In reactions (14), (15) radicals R• are expended, which further will not participate in the reaction of chain continuation (7). But since the concentration of radicals R• is far less than the concentration of dissolved oxygen (if oxygen enters the sample), the rate of reactions (14), (15) is far less than the reaction rate (2) and they have no effect on chemiluminescence intensity. Therefore, further, when analyzing the factors influencing the luminescence yield, we studied the reaction (6)–(9) only.
To estimate the dependence of chemiluminescence intensity in Fenton’s reaction at different substrate concentrations on the rate constants of some stages of chain oxidation, we calculated the S/S0 ratio: the relationship of light sums of the test substance S to the light sum of a blank Fenton’s solution S0 (Tables 1–4). Each table shows one of the constant varying greatly, while other constants were chosen as the most characteristic. Maximum sample concentration 100 mol/L was chosen on the principle that the lightest organic substances cannot have the concentration higher than this value. In case of a high-molecular substance (protein, e.g., albumin), molar concentration of protein (albumin), molecular weight being M~69 kDa, is not the concentration of oxidable substance [RH]. Each protein molecule has many enzymes, which can oxidize, and their concentration is far higher than the molar concentration of the protein itself.
In Table 1 the reaction rate constant (6) — k1 varies in the range from 102 to 1010 L(mol·s)–1. The represented findings show that if k1=102 there is no luminescence at all, with k1 growth the luminescence appears and increases. Maximum luminescence is achieved at the sample concentration, which is not maximum but significantly lower. Luminescence is evident if the sample concentration is 10–4–10–5 mol/L, and luminescence is more intensive than it is at the highest possible concentration. If k1 constant grows, the luminescence peak shifts towards lower sample concentrations, luminescence yield increasing.
Table 2 shows the calculated dependence of luminescence on the reaction rate constant (7) — k2. Luminescence does not occur if the rate constant is low, and the luminescence increases with k2 growth. At larger k2 luminescence may not achieve its maximum, and luminescence monotonically increases with the concentration growth [RH].
Table 3 shows the calculated values S/S0 at different reaction rate constant values (8) — k3. The luminescence is maximal at minimum k3 value. This is due to the fact that the reaction (8) results in hydroperoxide with no luminescence. With k3 growth, luminescence decreases and disappears. It should be emphasized that if k3 is relatively high, peroxidation proceeds, and hydroperoxide ROOH accumulates, though no luminescence occurs, and therefore, it cannot be recorded.
Table 4 demonstrates the calculation data for the reaction (9) — k4. The resultant of the reaction is the basic luminescent agent — singlet oxygen. Therefore, luminescence increases with k4 growth.
The process of chemiluminescence in Fenton’s reaction can be characterized as follows. In most cases, each test substance has the concentration, at which maximum luminescence occurs. As Tables 1–4 show, if constants k1–k4 vary over the wide range, chemiluminescence intensity changes greatly, the maximum position changing as well. With concentration increase of the substance in a sample (after maximum) luminescence decreases and can be not recorded at all. If the concentration decreases below the maximum, luminescence can also reduce. The absence of luminescence cannot indicate that the substance is inert, since at other concentrations, even lower ones, luminescence can appear (See Tables 2–4). The concentration values, at which luminescence reaches its maximum, are very much different. In our work we studied test luminescence in different dilutions in experiment. It can be said that for the mixture of substances with different molar concentration (different molecular weight) maximum luminescence can be observed for substances with large molecular weight (low molar concentration) at less dilution, than the substance with relatively low molecular weight and high molar concentration.
Thus, luminescence both in Fenton’s solution, and also in a biological sample has been revealed to be due to singlet oxygen dimer, which is the resultant of the reaction of radicals ROO•. Therefore, the presence of chemiluminescence of a biological sample in Fenton’s reaction determines the fact of radicals ROO• formation, as well as the fact that they interact among themselves, and other reactions of expending radicals ROO•, which result in no luminescence, do not expend all these radicals. Different S/S0 values when diluting samples (concentration decrease) till the dilution value when luminescence is maximum means the samples have different rate constants — k1–k4. No luminescence can mean that the sample oxidation does not result in luminescent product formation, their formation rate is low, or radicals ROO• die in reactions, which do not lead to luminescent product formation. Hydroperoxides still can be formed. We cannot assert that there is no oxidation, since there are very few substances, which are not oxidized by hydroxyl products. No hydroperoxides ROOH, which can be dentified using chemical methods, is the direct evidence of the fact there is no peroxidation.
It follows from what has been said that it is incorrect to use only such indices of Fenton’s reaction induced chemiluminescence, as S and Imax, with arbitrary concentration of a samle, to characterize the intensity of lipid peroxidation or free radical process intensity in a biological substrate.
Since a chain reaction induced by Fenton’s reaction results in hydroperoxides ROOH formation, and luminescence occurrence, substrate oxidability is characterized by the concentration of accumulated hydroperoxides and a recorded light sum; however, light sum alone is unable to assess completely the substrate oxidability.
Ferrous iron consumption and radiation pulse shape. Using the same program [8] we calculated the concentrations of the components participating in the reaction, and luminescence pulse form in case of different rate constants values of chain oxidation reactions. Recording time was 60 s. Fig. 2, а demonstrates the main reagent (Fe2+) consumption. Iron concentration over 60 s is shown to decrease by ~10 times, i.e. 60 s later initiation rate (the formation of radicals OH•) is an order less. The calculated forms of luminescence pulses are shown in Fig. 2, b and 1, c. It is obvious that there can be no luminescence intensity fall over 30 s, during which chemiluminescence is recorded experimentally (Fig. 2, c). Luminescence pulse characteristics (pulse leading-edge time, maximum luminescence intensity, position and width of maximum) are determined by reaction rate constants k1–k4.
Fig. 3 shows an experimental pulse form registered by a biochemiluminometer for different samples at the concentrations consistent with maximum luminescence. The emission of a blank Fenton’s solution without a biological sample terminates within 30 s (See Fig. 1, c). If there is a biological sample, luminescence does not always stop within 30 s. In certain cases, e.g. for albumin and blood plasma, luminescence intensity remains at the level of 60–70% of maximum. It is possible, when luminescence levels off 30 s later (See Fig. 2, c). Therefore, it turns to be impossible to relate the characteristics of a trailing edge of luminescence pulse with oxidation process characteristics.
Based on the experimental findings and the calculation data of Fenton’s reaction induced luminescence kinetics, it is incorrect to use a slope ratio of the trailing edge of chemiluminescence pulse, which is used in some studied [3, 4] as a criterion of an oxidizing ability.
Which Fenton’s reaction-induced chemiluminescence parameter characterizes an oxidation capacity of a test substance? When studying chemiluminescence induced by Fenton’s reaction we calculate light sum S for a test substance. To exclude the factors related to equipment sensitivity, we are to divide the light sum S by the light sum S0 of a blank Fenton solution, where a dissolving agent (water, phosphate buffer, saline solution, Hank’s solution, etc.) is used as a sample. S0 characterizes the device condition and Fenton’s reaction (iron solutions and hydrogen dioxide), and theoretically, in the process of an experiment these parameters do not change. That is why in the process of chemiluminescence study it is necessary to analyze S/S0 relationship. Fig. 4, а represents S/S0 values for albumin at an initial concentration 50 g/L and those for serial albumin dilutions. The presented findings show maximum S/S0 is reached when diluted –1 (ten times). When dilution is increased, light sum decreases. For a basic substance, which concentration 10 times as much, as 10–1 dilution, light sum value is lower.
Let us consider the dependence of light sum on the concentration of a hypothetic substance RH (Fig. 4, e). In this case, the reaction rate constant values are the following: k1=106, k2=105, k3=10, k4=104 L(mol·s)–1.
Initial concentration [RH]=100 mol/L. Maximum light sum is the resultant of the concentration, which 100 times less than the initial one (dilution: –2).
Suppose that there is one multi-component biological substrate, its light sum being Sx1, and another multi-component biological substrate with light sum Sx2. The light sums are different, but what can we say about the oxidation capacity of the samples? On account of calculations and experimental data we know nothing, since comparing only Sx1 and Sx2 values, it is impossible to learn what substrate concentration corresponds to maximum possible chemoluminescence light sum. Fig. 4 (a–e) shows that if the concentration of the substance in the sample increases, light sum at first may grow reaching its maximum, and then it decreases. In certain cases it can appear to be lower than the light sum of a blank Fenton solution, S/S0<1 (See Tables 2–4). Using the chemiluminescence indices of a test biological sample at different concentrations, we can obtain the concentration, at which the light sum is maximum. Maximum light sum for biological and organic substrates depending on a substrate condition (reaction rate constants k1–k4) can be reached at different concentrations (dilutions) of a substance (See Tables 1–4). Therefore, substance oxidation capacity is characterized by the concentration, at which maximum light sum is reached, and the value of its light sum.
Conclusion. The conducted research enables to conclude the following:
1. Optimal concentrations of hydrogen dioxide and ferrous iron to record chemiluminescence induced by Fenton’s reaction using a biochemiluminometer (BKhL-07) are: [H2O2]=10–3 mol/L, [Fe2+]=10–3 mol/L if pH=2.
2. Ferrous iron solution should be prepared in an acidic medium, pH=2 (with sulphuric acid added), since in a neutral medium iron is rapidly oxidized by atmospheric oxygen.
3. Chemiluminescence induced by Fenton’s reaction for both distilled water, and a biological sample, is due to singlet oxygen dimer decomposition and occupies the red spectral area.
4. S/S0 of a test multi-component biological substrate has maximum luminescence at certain concentration (dilution).
5. The position of maximum luminescence and maximum light sum is determined by rate constant values of chain initiation, continuation and disconnection reactions k1–k4.
6. The measurement of chemiluminescence in Fentonn’s reaction enables to assess oxidability of a test sample. It is incorrect to use such parameters as: maximum luminescence intensity (Imax), and a slope ratio of the trailing edge of chemiluminescence pulse (tga) as criteria of an oxidizing ability of a substance, if concentration value is arbitrary chosen.
7. The characteristic of test substance oxidability when studying chemiluminescence induced by Fenton’s reaction is the concentration (dilution), when maximum light sum S/S0 is recorded.
Study Funding and Conflict of Interests. The study was not funded by any sources, and there are no conflicts of interest related to the present study.
References
- Vladimirov Yu.A., Proskurina E.V. Free radicals and cell chemiluminescence. Uspekhi biologicheskoy khimii 2009; 49: 341–388.
- Alyasova A.V., Kontorshickova K.N., Terentiev I.G., Ivanova I.P., Kuznetsov S.S., Sazanov A.I. Influence of the ozonized physiologic salt solution low therapeutic concentrations on a tumor therapeutic pathomorphosis in experiment. Sovremennye tehnologii v medicine 2010; 4: 27–32.
- Borovkova L.V., Rassohin V.F., Shcherbatyuk T.G., Kolobova S.O. Influence of SCANNER-therapy on general anti-oxidant and free-radical blood activity in case of noncarrying of pregnancy of infectious genesis. Medicinskij al’manah 2010; 2: 179–181.
- Maslennikova A.V., Shcherbatyuk T.G., Lazareva V.A., Davyidenko D.V. Prognostic value of parameters of pro-oxidant and antioxidant status among patients with locally advanced intraoral and pharynx cancer. Medicinskij al’manah 2009; 3: 110–113.
- Alekhina S.P., Shcherbatyuk T.G. Ozonoterapiya: klinicheskie i eksperimental’nye aspekty [Ozone therapy: clinical and experimental aspects]. Nizhny Novgorod: Izd. “Litera”; 2003.
- Syrkin A.M., Zorina L.N. Klassifikatsiya i nomenklatura neorganicheskikh veshchestv [Classification and nomenclature of inorganic substances]. Ufa: UGNT; 2006.
- Vladimirov Y.A., Proskurnina E.V., Izmajlov D.Y. Kinetic chemiluminescence as a method for study of free radical reactions. Biophysics 2011; 56(6): 1055–1062, http://dx.doi.org/10.1134/S0006350911060200.
- Ivanova I.P., Trofimova S.V., Piskarev I.M., Aristova N.A., Burkhina O.E., Soshnikova O.O. Mechanism of chemiluminescence in Fenton reaction. Journal of Biophysical Chemistry 2012; 3(1): 88–100.
- Ivanova I.P., Trofimova S.V., Piskarev I.M. Evaluation of prooxidant properties of ascorbic acid. Biophysics 2013; 58(4): 453–456, http://dx.doi.org/10.1134/S0006350913040076.
- Tsaplev Y.B. Statistical analysis of recording light emitted by ZhS-19 glass. Optika i spektroskopiia 2008; 2(104): 317–318.
- Ermolin S.V., Ivanova I.P., Knyazev D.I., Trofimova S.V., Piskarev I.M. Mechanism of water luminescence upon radiolysis under the effect of background radiation. Russian Journal of Physical Chemistry A 2012; 86(6): 1029–1032, http://dx.doi.org/10.1134/S003602441206009X.
- Zhen Lin, Hui Chen, Yun Zhou, Nobuaki Ogawa, Jin-Ming Lin. Self-catalytic degradation of ortho-chlorophenol with Fenton’s reagent studied by chemiluminescence. J Environ Sci 2011; 23: 1–24, http://dx.doi.org/10.1016/S1001-0742(10)60639-0.
- Aristova N.A., Ivanova I.P., Trofimova S.V., Knyazev D.I., Piskarev I.M. Influence of luminol on the chemiluminescence intensity in Fenton’s reaction. High Energy Chemistry 2011; 45(6): 505–509, http://dx.doi.org/10.1134/S0018143911060038.
- Aristova N.A., Ivanova I.P., Trofimova S.V., Knyazev D.I., Piskarev I.M. Luminol-dependent luminescence accompanying Fenton’s reaction. Issledovano v Rossii 2011; р. 77–86, http://www.sci-journal.ru/articles/2011/008.pdf.
- Schaich K.M. Lipid oxidation: theoretical aspects. 6th ed. New York: John Wiley & Sons; 2005, http://dx.doi.org/10.1002/047167849X.bio067.
- Ivanova I.P., Piskarev I.M., Trofimova S.V. Initial stage of lipid peroxidation with HO2• radicals. Kinetic study. American Journal of Physical Chemistry 2013; 2(2): 44–51, http://dx.doi.org/10.11648/j.ajpc.20130202.13.


















