Brain Cancer Immunotherapy (Review)
The review analyzes Russian and foreign reports concerned with a rapidly developing brain cancer treatment technique — immunotherapy. There has been presented a current view on the basic concept of antitumor immunity, on the problem of immune system interaction with a tumor in general and under the conditions of an immunologically privileged nervous system, shown the theoretical background of efficiency of immunotherapy used against brain cancer (the capability of tumor antigens and activated lymphocytes to penetrate the blood-brain barrier). There has been demonstrated the role of a transforming growth factor β, interleukin 10, cyclooxygenase-2, prostaglandin Е2, protein MCP-1, interactions Fas-receptor/Fas-ligand, antigen-4 cytotoxic Т-lymphocytes in tumor immunoresistance development. The review presents a current classification of the types of active and passive immunotherapy, each of the types being considered separately specifying the characteristics, the results of preclinical and clinical trials of each type efficiency, and possible side effects. Special attention has been paid to a new concept of a key role of tumor stem cells in the pathogenesis of cerebral gliomas and the target action on these cells.
Results of the traditional approach in treatment of malignant brain tumors with median survival in patients diagnosed with glioblastoma (Grade IV) and with the 2-years survival rate equal to 26.5% can not be called satisfactory [1]. Choice of a certain treatment method is performed, primarily, based on histological results of tumors, which differentiate tumor cells from the normal ones at the macroscopic level, as well as presence of cellular or nuclear polymorphisms, endothelial proliferation, mitosis, necrosis and vascular thrombosis [2]. However, there are many aspects which remain an open question, i.e. why in certain cases we observe the positive response to the complex therapy, and in other cases, this response is minimal. Nowadays we can speak about a certain progress in studying biology of different malignant tumors, including brain tumors. Contemporary research is dedicated to studying pathogenetic mechanisms of tumor growth at molecular and genetic levels, which allows to detect principle differences of tumors with identical histological patterns. The said differences may contribute to anticipation of life expectancy periods. At present recommended treatment protocols already include new molecular and genetics prognosis criteria, i.e. presence of O-6-methylguanine-DNA methyltransferase (MGMT), deletions 1p/19q, and IDH1/IDH2 mutations [3].
Success in studying of malignant tumors nature and clinical significance of detected molecular and genetic characteristics of a tumor predetermine search for some fundamentally new treatment methods, based on selective affection of cells which are genetically different from the normal body cells. One of such methods is immunotherapy, which is based upon activation and strengthening of processes related to the specific immune response of the body to the tumor growth. There have been reported certain successful results in immunotherapy of oncological diseases. The US FDA has approved for the wide clinical use approximately 17 immunotherapeutic remedies [4]. Scientists are constantly working on different options of immunotherapeutic influence upon glial brain tumors, with many of the said options successfully passing stages I and II of clinical trials. However, due to the small scale of the studies which have even managed to deliver hopeful results, evidential base of the method is still insufficient.
This review covers contemporary points of view to the anti-tumor immunity, pathogenesis of gliomas and their interaction with the immune system of the body, as well as primary possible types of immunotherapy.
Concepts of antitumor immunity
The primary prerequisite for development of the immunotherapeutical method is the state of having sufficient biological defenses to avoid any genetically foreign information, called immunity. The basic idea of the method is to regulate universal and natural mechanisms of the immune system, which allows, to the certain degree of accuracy, to speak about possible specificity and adjusted to human physiology. Alterations in cells genome during the process of tumor transformation triggers activation of antitumor immunity. The immunosurveillance theory suggests that T and Nk cells constantly control over antigen content of self cells of the body with elimination of the latter when new antigenes (AG) are being detected on their surface. On tumor cells geterogenic (testicular cancer), differential (tissue-specific), mutant and other AGs have been detected (Fig. 1). Detected in gliomas MAGE-1 and SOX6 are classified as testicular cancer AGs, which are normally being synthesized in embryos and in gonads of adults [5, 6]. Tissue-specific AGs, i.e., the ones detected in gp100 and TRP-2 gliomas, are proteins, normally synthesized by brain-tissue at certain stages of its development [7, 8]. Mutant IGFRvIII AG is often detected in glioma cases [9]. A great number of other AGs specific for gliomas have been identified as well, i.e.: IL13Ra2 [10], EphA2 [11], EphB6 [12], AIM-2 [13], HER-2 [8], WT1 [14], ARF4L [15], SART-3 [16], SOX11 [17], KIF1 and KIF3C [18].
 Fig. 1. Primary types of glial tumor antigens Fig. 1. Primary types of glial tumor antigens
|
The immunosurveillance theory is confirmed by increased numbers of serum antibodies, infiltration of tumor tissue by lymphocytes, by the high rate of tumor development caused by long-term use of immunodepressants.
However it has been confirmed, that the immune system can contribute to tumor progression as well, by participating in development of tumor immunogenic phenotype. As a result of controversial role of the immune system a contemporary theory about complex relations between tumors and the body has been developed, this concept is internationally known as “the three Es of cancer immunoediting” theory [19]. This concept suggests three phases of relations between a tumor and the immune system, viz. elimination, equilibrium and escape (Fig. 2).
 Fig. 2. Theory of relations between “a tumor and the body”. “The three Es of cancer immunoediting” — elimination, equilibrium and escape Fig. 2. Theory of relations between “a tumor and the body”. “The three Es of cancer immunoediting” — elimination, equilibrium and escape
|
Elimination phase is synonymous with the concept of immunosurveillance which implements mechanisms of both the innate and adaptive immunity. At the equilibrium stage the immune system and a part of a tumor, which somehow escaped elimination, are in the state of dynamic equilibrium, which sustains active immune influence upon the tumor cells, although, this influence is insufficient to be able to achieve complete suppression of tumor growth. Tumor tissue continues to sustain processes of differentiation, new mutant cells keep appearing, resulting in genetic instability of the tumor, which can eventually cause development of a completely new population of tumor cells with lowered immunogenesis and increased resistance. Tumor cells, modified during the equilibrium phase, start active division, but this time being undetected by immunosurveillance mechanisms. The said processes allow us to consider the equilibrium phase as playing a key role in development of tumor resistance, which points out to the necessity of complete elimination of tumor cells at the phase of active immunosurveillance.
The most effective would have been immunity enhancement at the elimination and equilibrium phases, but unfortunately, patients with brain tumors come to the clinic usually at the escape stage, when the human immune system is not able to recognize and/or eliminate tumor cells. However the method of immunotherapy is still expected to bring positive results. This method is dedicated to activate antitumor mechanisms for the purpose of restoration of the active stage of the immune response in order to eliminate tumor cells disseminated in the brain upon operative removal of the major part of the tumor.
Immune system disorders as a result of malignant brain tumors
Phenomenon of the “immune privilege” of the CNS. The theory of the “immune privilege” of the CNS states that normal activities of the immune system in the CNS are prevented by anatomic and functional isolation resulting from the existence of the blood-brain barrier (BBB) [20]. The BBB is a highly organized system consisting of endothelial cells, which are connected by tight junctions: basement membrane, pericytes and astrocytes [21]. The said tight junctions connecting membranes of endothelial cells with high content of mitochondria, low levels of pinocytosis and absence of fenestra result in almost complete impenetrability of the BBB for hydrophiles [22]. It is common knowledge that the process of transplant rejection in the brain takes place much slower than in other organs [23].
In general, we may single out several factors, preventing normal activities of the immune system in the CNS, such as:
limited drainage of AGs from the CNS into the cervical lymphnodes [24];
inability of free penetration of native T cells and antibodies into the CNS [25];
insignificant number of native antigen-presenting cells in the CNS [24];
suppression of the functioning and rapid development of T cells apoptosis by means of activation of FasL and brain gangliosides [26];
absence of homing receptors for leukocytes in the CNS [24].
More recent evidence has led to remodeling of the immunosurveillance theory. It was discovered that macrophages, microglias [27, 28] and dendritic cells [28] are the major APCs in the CNS. There are two ways of penetrating into the lymphoid tissue for the foreign protein AGs: 1) along the olfactory nerve through lamina cribrosa into the lymphoid tissue of mucous membrane of nasal passages; 2) in the area of the ependymal lining of ventricles and Virchow–Robin spaces the intestinal fluid drains into the cerebrospinal fluid, which, in its turn, drains into the cervical lymphnodes [29]. At the same time, the activated lymphocytes may penetrate back through the BBB [30] and express tropic to the CNS integrins (i.e. α4β7 [31]) (Fig. 3):
 Fig. 3. Possible ways of activation of the immune response and penetration of activated T cells into the CNS in accordance with the CNS “immune privilege” theory Fig. 3. Possible ways of activation of the immune response and penetration of activated T cells into the CNS in accordance with the CNS “immune privilege” theory
|
from the choroid plexus of ventricles into the cerebrospinal fluid;
through Virchow–Robin spaces and postcapillary venules into the subarachnoid space;
lymphocytes may directly penetrate through the BBB into the cerebral tissue.
Despite of certain obstacles preventing realization of the immunosurveillance, the CNS has the entire range of afferent and efferent links of the immune system, which speaks for the possible application of the immunotherapeutical method for treatment of malignant brain tumors, subject to the contemporary knowledge of distinctive features of the immune system functioning in the CNS. For example, when applying the vaccine onto the dendritic cells, it is necessary to consider capability of the latter to activate a large number of native T cells, and, therefore, allowing them to freely penetrate the BBB.
Suppression of the immune system by a tumor. The stated above distinctive features of the immune system functioning in the CNS create promising prerequisites for the further development of immunotherapy, however, despite of a certain degree of success of experiments in mice, results of clinical trials remain unsatisfactory. At present, the following factors, have been determined as limiting the efficiency of the immune system and as contributing to the tumor resistance [32]:
1. Transforming growth factor β (TGF-β) is one of the most probable immunosuppressive cytokines which suppresses several significant antitumor mechanisms, i.e. maturation of APC and their activity; activation of T cells and their further differentiation [33].
2. Interleukin 10 (IL-10) synthesized by monocytes, Th2-lymphocytes, and regulatory T cells (Tregs). It reduces expression of Th1-cytokines, MHC molecules, and co-stimulatory APC molecules [34].
3. Cyclooxygenase-2 (COX-2) and synthesized with its help prostaglandin E2 (PGE2) promote invasive tumor growth and angiogenesis, maintain tumor resistance against activities of the immune system by inducing Tregs and suppressing Th1 cells [35].
4. Monocyte chemoattractant protein 1 (MCP1) or CCL2 with the angiogenic effect supports populations of immunosuppressive MDSC and Tregs leukocytes [36].
5. Fas receptor/Fas ligand (FasR/FasL). FasR (CD95, APO-1) is a surface receptor which launches apoptosis. Its secretion on tumor cells stimulates inflammation and angiogenesis, which support and protect tumor growth [37]. Moreover, glioblastomas express Fas ligands, which cause death of immune cells infiltrating a tumor.
6. Cytotoxic T-lymphocyte antigen-4 (CTLA-4 or CD152) is similar to CD28 molecule, which is expressed on activated T cells. CD28 is normally a receptor to B7 proteins, securing a strong co-stimulating signal. CTLA-4, secreted by regulatory T cells and possessing a higher level of affinity to B7, results in lymphocytic tolerance [38].
Many studies determined that a number of immune cells are not acting as immunosurveillance factors but, on the contrary, contribute to tumor growth [39–41]. Thus, MDSCs [42] and Tregs [43] express a significant number of factors, including the immunoregulatory ones, i.e. CD25, CTLA-4, GITR, CRCR4 [44], which may suppress activation, proliferation and functioning of T cells.
Gliomas successfully use mechanisms of immunosuppression for the further growth under conditions of insufficient activity of immune cells in peripheral blood. Tumor influence upon the immunity is evidenced by the presence in brain gliomas patients of significant qualitative and quantitative immune system disorders, primarily in cells, which lead to development of the secondary immunodeficiency [45, 46]:
— development of the general lymphocytopenia (CD3+, CD19+);
— significant decrease of protein levels (PLCγ1 and p56lck) in T cells, resulting in functional disorders of TCR/CD3, which take part in cooperation with APC;
— decrease of proliferative activity and disbalance of the major subpopulations of T cells (primarily caused by decrease of CD4+), decrease of functional activity of T-lymphocytes as a result of expression by B7-H1 glioma [47];
— quantitative decrease of CD4+ Т-lymphocytes; suppression of their sensibility to stimulation by mitogens (ConA, PHA, anti-CD3 mAb, etc.) and by antigenes result in decreased secretion of anti-inflammatory cytokines IL-2 and INFγ, necessary to develop cytotoxicity, associated by lymphokine-activated killers (LAK) and cytotoxic lymphocytes [48];
— decreased secretion of IL-2 and receptors to it leading to reduction of proliferative capacity and functional activity of CD8+ T-lymphocytes [49];
— disorder of humoral immunity resulting in quantitative decrease of immunoglobulins, complement system molecules and increase in number of circulating immune complexes;
— significant decrease of macrophages phagocytal activity with development of immunosuppressive phenotype M2, suppression of macrophage activation as a result of secretion of VEGF and Il-6 by tumor cells [47, 50, 51].
Active suppression by a tumor of the human immune system and its low functioning at the stage of cancelation of immunosuppressive mechanisms result in rapid tumor proliferation. Support of efficient functioning of the immune system and influence upon immunosuppressing factors has high potential for managing over uncontrolled tumor growth.
Immunotherapy of glial brain tumors
Immunotherapeutic procedures of malignant brain tumors can be divided in the passive and active ones (Fig. 4). Passive immunotherapy includes direct introduction of monoclonal antibodies (mAbs) and effector immune cells — lymphokine-activated killers or specific cytotoxic lymphocytes. Administration into the body of monoclonal antibodies is based upon their selective cooperation with tumor-associated AGs. Introduction of LAK and cytotoxic lymphocytes with the purpose of strengthening of the effector link of the anti-tumor response some authors identify as an individual branch of adoptive immunotherapy.
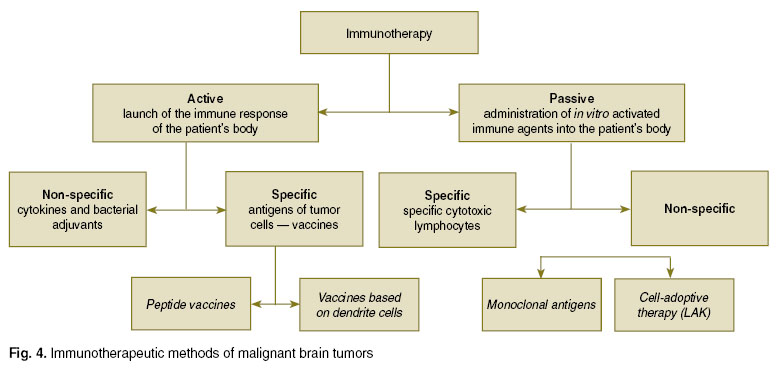 Fig. 4. Immunotherapeutic methods of malignant brain tumors Fig. 4. Immunotherapeutic methods of malignant brain tumors
|
Active immunotherapy presupposes stimulation of immunocompetent cells, performed by means of administration of cytokines, different proteins or dendrite cells. In case of specific active immunotherapy it is possible to use two types of vaccines: 1) based on tumor-associated AGs (peptide vaccines); 2) based on dendrite cells.
Passive immunotherapy. This method is about activation of ex vivo tumor AGs elements of the immune system with acquisition of tumor-associated antibodies and effector cells with their further administration to a patient’s body.
1. Monoclonal antibodies preparations. Efficiency of preparations of antitumor monoclonal antibodies depends on many factors, including stability and availability of AGs, density of their distribution inside a tumor. Under normal conditions the BBB is impenetrable for antibodies, but endothelial cells of neoplastic tumor vessels are loosely connected, which allows antibodies to penetrate into the tumor. Successful use of antibodies marked by a radioactive isotope for visualization of tumors confirms this thesis [52]. New methods of the BBB penetrating enhancement with the use of bradykinin agonists, focused ultrasound, ozonized saline, etc. [53–55].
At present there are many potentially efficient antigen preparations. For example, it is possible to use monoclonal antibodies (BC-2 and 81C6) to tenascin — the adhesive tumor protein, spread, primarily, in extracellular matrix and perivascular space of gliomas [56, 57]. The distinctive feature of glial tumors is hyper-expression of transmembrane receptor of the epidermal growth factor receptor (EGFR), fixation of which leads to decrease of vascularisation, proliferative activity and apoptosis of tumor cells [58]. There is an entire range of monoclonal antibodies medical drugs, aimed against EGFR. For example, cetuximab (Erbitux) — a chimeric monoclonal antibody to EGFR. Preclinical studies proved efficiency of this drug, although the II phase of the clinical trial showed no visible therapeutic effect [59]. A possibility of use of monoclonal antibodies to EGFR trastuzumab (Herceptin) and panitumumab (Vectibix) was studies, primarily, on patients with breast and rectal cancel, so there is an insignificant number of studies devoted to efficiency of this antibodies in treatment of gliomas [60]. Bevacizumab is recombinant humanized monoclonal antibodies, which selectively associate with the vascular endothelial growth factor A (VEGF-A). Efficacy of the drug in case of recurrent glioblastoma, which resulted in increased survival rate of patients and decrease of tumor recurrence, was proven during the II phase of clinical trials [61–64]. A conducted meta-analysis of trials devoted to the use of bevacizumab in combination with irinotecan showed increase of non-recurrence period, although, it did not confirm any significant increase of survival rate [65].
Preclinical trials of daclizumab [66] — a preparation of antibodies aimed against IL-2Rα (CD25), expressed by immunosuppressing T-regulative cells, have been conducted. At present a possibility of its use in complex immunotherapy of gliomas is being investigated [67].
2. Cellular adoptive immunotherapy. In natural anti-tumor immunity the key role belongs to its cellular link. The first experience of using cellular passive immunotherapy was related to the local administration of autological LAK and recombinant IL-2 into the tumor bed prior and upon surgery [68–70]. This methods has a number of side-effects, including cerebral edema, increased blood pressure, headaches, fever and depression of consciousness. Most of the studies have not reveal any increase of the average survival rate of patients, although, a number of works consider this method as effective in glioblastoma treatments [70, 71], however it is necessary to mention that LAK has non-specific cytotoxicity, i.e. these cells are active strictly against tumor cells.
A more promising method seems to be the use of ex vivo tumor AG-activated mononuclear lymphocytes of peripheral blood; promoted by 1) high specificityof detection by T-lymphocytes of tumor cells; 2) the capability of activated T-lymphocytes to easily penetrate the BBB. G.E. Plautz et al. [72] conducted the study on patients with brain glial tumor. These patients were administered with irradiated autological tumor cells, mixed with granulocyte-macrophage colony-stimulating factor (GM-CSF), and then activated T-lymphocytes were taken out from the draining lymphnode and stimulated by the bacterial superantigen — staphylococcal enterotoxin A and, in certain cases, by anti-CD3. 2 of 10 patients showed regress of glioblastoma recurrence based on neurovisualization data.
Active immunotherapy
1. Dendritic-cell-based therapeutic cancer vaccines. Despite the capability of tumor cells to present AGs against T-lymphocytes, the immune response, caused by them is very weak. Most probably, this is related to inefficiency of AG presentation and insufficiency of costimulating molecules. The human body has professional antigen-presenting cells — the dendritic cells, which possess high capabilities to present foreign antigens to T-lymphocytes due to the high expression on them of MHC molecules and MHC complexed with costimulating CD80 and CD86 molecules. We may distinguish two primary subpopulation of dendritic cells — DC1 and DC2, which differentiate by histogenesis (myeloid and lymphoid), by cellular phenotypes (CD11ch1-CD123Io and CD11c-CD123hi accordingly) and by influence upon T-lymphocytes (activation of the immune system and energy induction, respectively). Dendritic cell are capable to activate resting, and what’s primarily important, native T-lymphocytes. In cultures, there are two way of acquisition of myeloid dendritic cells [73]: 1) from bone marrow cells, enreached by CD34+ stem elements in the presence of GM-CSF and other cytokines (more often TNF-α, sometimes IL-3, SCF, FLT3L or TGFβ); 2) out of monocytes in the presence of GM-CSF and IL-4. By now a great number of preclinical trials has been conducted. During these trials multiple methods of loading the dendritic cells by different AGs have been used. Sources of tumor AGs may be: tumor lysate containing inactivated by ultrasound tumor cells, synthetic or acid-eluted peptides, tumor RNA and DNA. At present the method of use of dendritic cells is widely studies during clinical trials for all kinds of tumors, i.e. melanomas, renal carcinomas and prostatic cancer.
A clinical trial devoted to the use of loaded by tumor AGs dendritic cells resulted in a significant increase of patients’ survival rate. Median survival of glioblastoma patients in the immunotherapy group with the use of dendritic cells and in the control group was 455 and 257 days, respectively [74]. During this study surface tumor AGs were acid-eluted and incubated with autologous dendritic cells, acquired from monocytes of peripheral blood in the presence of GM-CSF and IL-4. It is necessary to mention, that this method did not result in any significant side-effects or autoimmune toxicity. During some other clinical trial the dendritic cells were incubated with autologous tumor cells in the presence of polyethyleneglycol [75]. The study resulted in a partial treatment response and no significant side-effects. A recent trial [76], involving 77 patients diagnosed with a glioblastoma for the first time, also resulted in no side-effects related to the use of the vaccine. Thus, use of dendritic-cell-based therapeutic cancer vaccines may be called conditionally safe. Although, it is difficult to evaluate efficiency of this immunotherapeutic method due to differences in methods of vaccine application and designs of the trials. A choice of an optimal source of dendritic cells, inductive methods of their maturation, methods of loading by tumor cells, the most efficient vaccination protocols, and use of cytokines as adjuvants remain open-ended questions [77].
2. Peptide vaccines. Use of these vaccines is a very perspective type of immunotherapy of malignant brain tumors. APC present these AGs together with the MHC molecule, thus, activating cytotoxic lymphocytes. Results of many trial devoted to efficiency of peptide vaccines against all kinds of malignant tumors can be called unsatisfactory [78]. Although, the most recent studies of these vaccines against malignant gliomas showed some positive results.
Therapy of glioblastoma patients using peptide vaccines against EGFRvIII protein, mutant form of EGFR, has successfully passed stage I of VICTORI clinical trials, during which patients were administered with dendritic cells, loaded with EGFRvIII, conjugated with KLH (Keyhole limpet hemocyanin) [79]. Median survival of patients receiving the vaccine was 22.8 months, which is significantly more, than in patients, receiving temozolomide (14.6 months) [1]. A recent II stage study of 21 patients with EGFRvIII-positive glioblastomas, showed significant increase of the median survival (26 months, p=0.0013) with the use of the vaccine against EGFRvIII vs. the control group, which did not receive the immunotherapy [80]. During the study, patients upon undergoing surgery, standard radiation and chemotherapy with temozolomide, were receiving on a monthly basis the vaccine against EGFRvIII until tumor progression; the medium time until tumor progression was 16.6 months. At present the III stage of the clinical trials devoted to the use of vaccines based on EGFRvIII protein is being planned to be carried out.
Since immunogenisity of tumor AGs can be weak, certain attempts are being made in order to use a mixture of different antigens for the vaccination purposes [81]. By use of this strategy it is possible to receive a strong and rapid response resulting in development of cytotoxicity determined by cytotoxic lymphocytes [82]. Data of a stage I clinical trial related to research of polyepitope vaccines used in 25 patients with malignant brain gliomas has been published [81]. The trial involved prevaccination with further sampling of mononuclear lymphocytes of peripheral blood and plasm from patientsfor the purpose of analyzing the body response to the vaccine. The most immunologically relevant proteins, such as lymphocyte-specific protein tyrosine kinase and multidrug resistance-associated protein 3 have been determined. From 5 percent of patients a partial response has been acquired, the median survival was 18 months. A recent clinical trial related to research of vaccinations using individual peptide cocktails has brought some positive results as well [83].
Immunotherapy aimed against tumor stem cells
Currently, a new theory of a key role of tumor stem cells in pathogenesis of glial brain tumors is being widely discussed. According to this theory the tumor tissue has a pool of stem cells responsible for tumor proliferation, high invasiveness, resistance to radiation and chemotherapy, migration of tumor cells far out from the primary site, provided however that the other part of the tumor cells is not actively participating in these processes and plays a secondary role [84–86]. Tumor stem cells express on their surface a number of typical CD133, Nestin, Sox2, CD15, Musachi molecules, and use them for the identification purposes [87]. Presence in tumor stem cells of multidrug resistance genes (MDR-genes) [88], allows them to not only response to chemotherapy drugs but to recover tumor growth [89]. Most probably, tumor stem cells are more resistant to radiation therapy as well [90]. One of the key factors of resistance to radiation and chemotherapy is the tumor vasculature, as subject to disorders of the BBB in tumor tissue and due to increase of interstitial pressure transportation of medical drugs to the tumor cells is hindered [91, 92]. At the same time, radiation therapy increases expression of VEGF in tumor cells [93], which is the primary growth factor of tumor vessels, and which may contribute to tumor growth and, probably, resistance to chemotherapy.
Thus, taking into consideration precedence of radiation and chemotherapy aimed against “auxiliary” tumor cells, it is necessary to continue searching for new effective methods to affect tumors by means of targeting tumor stem cells.
A possible option of targeting tumor stem cells is immunotherapy. SOX6 — specific AG for glioma-associated stem cells and gliomas in general [94]. Derivatives of SOX6 — antigenes of A2 and A24 leukocytes have been detected. Use of these derivatives resulted in stimulation of human lymphocytes, causing specific T cell response [95]. Another antitumor stem cells immunotherapeutic method is the use of dendritic cell-based vaccines with tumor stem cells, derived from tumor material. Some currently conducted clinical trials studying glial tumors in mice and rats have shown these vaccines with tumor stem cells are promising [96, 97]. Probably, tumor stem cells in particular shall be the primary target when developing treatment methods of malignant brain tumors.
Conclusion
Immunotherapy of malignant brain tumors is a perspective treatment method, based upon activation and strengthening of self body defenses. Actions of the immune system are characterized by the high specificity, a capacity to control antigene content of body cells in general, absence of a significant toxic effect related to healthy cell. Efficiency of the method is limited by certain immunological isolation of the CNS, by the tumor capacity to suppress immune system protective mechanisms, formation by the tumor of vascular and stromal membranes, and presence of tumor stem cells with high mutative capacities. At present, any existing results of immunotherapy of glial brain tumors are far from being perfect. However further studies in the field of resistance mechanisms of gliomas against different treatment approaches, new data on tumor nature and relationships in the “tumor–organism” system shall become a foundation for development of new strategies in immunotherapy of malignant brain tumors, which allows to be positive about improving results in treatment of patients with malignant brain tumors.
Study Funding and Competing Interest. This study was not supported by any financial sources and there is no topic specific conflict of interest related to the authors of this study.
References
- Stupp R., Mason W.P., van den Bent M.J., et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 2005; 352(10): 987–996, http://dx.doi.org/10.1056/NEJMoa043330.
- Olyushin V.E. Cerebral gliomas: a brief review and a treatment protocol. Neyrokhirurgiya 2005; 4: 41–47.
- Konovalov A.N., Potapov A.A., Olyushin V.E., et al. Standards, options and recommendations in the management of primary CNS tumors (2013–2014). Moscow; 2013.
- Dillman R.O. Cancer immunotherapy. Cancer Biother Radiopharm 2011; 26(1): 1–64, http://dx.doi.org/10.1089/cbr.2010.0902.
- Kuramoto T. Detection of MAGE-1 tumor antigen in brain tumor. Kurume Med J 1997; 44(1): 43–51, http://dx.doi.org/10.2739/kurumemedj.44.43.
- Ueda R., Yoshida K., Kawakami Y., et al. Expression of a transcriptional factor, SOX6, in human gliomas. Brain Tumor Pathol 2004; 21(1): 35–38.
- Chi D.D., Merchant R.E., Rand R., et al. Molecular detection of tumor-associated antigens shared by human cutaneous melanomas and gliomas. Am J Pathol 1997; 150(6): 2143–2152.
- Liu G., Ying H., Zeng G., et al. HER-2, gp100, and MAGE-1 are expressed in human glioblastoma and recognized by cytotoxic T cells. Cancer Res 2004; 64(14): 4980–4986, http://dx.doi.org/10.1158/0008-5472.CAN-03-3504.
- Heimberger A.B., Crotty L.E., Archer G.E., et al. Epidermal growth factor receptor VIII peptide vaccination is efficacious against established intracerebral tumors. Clin Cancer Res 2003; 9(11): 4247–4254.
- Okano F., Storkus W.J., Chambers W.H., et al. Identification of a novel HLA-A*0201-restricted, cytotoxic T lymphocyte epitope in a human glioma-associated antigen, interleukin 13 receptor alpha2 chain. Clin Cancer Res 2002; 8(9): 2851–2855.
- Hatano M., Eguchi J., Tatsumi T., et al. EphA2 as a glioma-associated antigen: a novel target for glioma vaccines. Neoplasia 2005; 7(8): 717–722.
- Jin M., Komohara Y., Shichijo S., et al. Identification of EphB6 variant-derived epitope peptides recognized by cytotoxic T-lymphocytes from HLA-A24+ malignant glioma patients. Oncol Rep 2008; 19(5): 1277–1283, http://dx.doi.org/10.3892/or.19.5.1277.
- Liu G., Yu J.S., Zeng G., et al. AIM-2: a novel tumor antigen is expressed and presented by human glioma cells. J Immunother 2004; 27(3): 220–226.
- Hashiba T., Izumoto S., Kagawa N., et al. Expression of WT1 protein and correlation with cellular proliferation in glial tumors. Neurol Med Chir (Tokyo) 2007; 47(4): 165–170; discussion 170, http://dx.doi.org/10.2176/nmc.47.165.
- Nonaka Y., Tsuda N., Shichijo S., et al. Recognition of ADP-ribosylation factor 4-like by HLA-A2-restricted and tumor-reactive cytotoxic T lymphocytes from patients with brain tumors. Tissue Antigens 2002; 60(4): 319–327, http://dx.doi.org/10.1034/j.1399-0039.2002.600406.x.
- Murayama K., Kobayashi T., Imaizumi T., et al. Expression of the SART3 tumor-rejection antigen in brain tumors and induction of cytotoxic T lymphocytes by its peptides. J Immunother 2000; 23(5): 511–518.
- Schmitz M., Wehner R., Stevanovic S., et al. Identification of a naturally processed T cell epitope derived from the glioma-associated protein SOX11. Cancer Lett 2007; 245(1–2): 331–336, http://dx.doi.org/10.1016/j.canlet.2006.01.014.
- Harada M., Ishihara Y., Itoh K., Yamanaka R. Kinesin superfamily protein-derived peptides with the ability to induce glioma-reactive cytotoxic T lymphocytes in human leukocyte antigen-A24+ glioma patients. Oncol Rep 2007; 17(3): 629–636, http://dx.doi.org/10.3892/or.17.3.629.
- Dunn G.P., Old L.J., Schreiber R.D. The three Es of cancer immunoediting. Annu Rev Immunol 2004; 22: 329–360, http://dx.doi.org/10.1146/annurev.immunol.22.012703.104803.
- Muldoon L.L., Alvarez J.I., Begley D.J., et al. Immunologic privilege in the central nervous system and the blood-brain barrier. J Cereb Blood Flow Metab 2013; 33(1): 13–21, http://dx.doi.org/10.1038/jcbfm.2012.153.
- Chekhonin V.P., Baklaushev V.P., Yusubalieva G.M., et al. Fundamental and application aspects of blood-brain barrier study. Vestnik Rossiyskoy Akademii meditsinskikh nauk 2012; 8: 66–78.
- Bechmann I., Galea I., Perry V.H. What is the blood-brain barrier (not)? Trends Immunol 2007; 28(1): 5–11, http://dx.doi.org/10.1016/j.it.2006.11.007.
- Tambur A.R. Transplantation immunology and the central nervous system. Neurol Res 2004; 26(3): 243–255, http://dx.doi.org/10.1179/016164104225013932.
- Anirban G. Immune connection in glioma: fiction, fact and option, glioma, in glioma — exploring its biology and practical relevance. In: Glioma — exploring its biology and practical relevance. Ed. by Ghosh D.A. InTech; 2011; р. 305–324.
- Hickey W.F. Leukocyte traffic in the central nervous system: the participants and their roles. Semin Immunol 1999; 11(2): 125–137, http://dx.doi.org/10.1006/smim.1999.0168.
- Flügel A., Schwaiger F.W., Neumann H., et al. Neuronal FasL induces cell death of encephalitogenic T lymphocytes. Brain Pathol 2000; 10(3): 353–364.
- Yang I., Han S.J., Kaur G., et al. The role of microglia in central nervous system immunity and glioma immunology. J Clin Neurosci 2010; 17(1): 6–10, http://dx.doi.org/10.1016/j.jocn.2009.05.006.
- Karman J., Ling C., Sandor M., et al. Dendritic cells in the initiation of immune responses against central nervous system-derived antigens. Immunol Lett 2004; 92(1–2): 107–115, http://dx.doi.org/10.1016/j.imlet.2003.10.017.
- Goldmann J.E., Kwidzinski C., Brandt C., et al. T cells traffic from brain to cervical lymph nodes via the cribroid plate and the nasal mucosa. J Leukoc Biol 2006; 80(4): 797–801, http://dx.doi.org/10.1189/jlb.0306176.
- Ransohoff R.M., Kivisakk P., Kidd G. Three or more routes for leukocyte migration into the central nervous system. Nat Rev Immunol 2003; 3(7): 569–581, http://dx.doi.org/10.1038/nri1130.
- Calzascia T., Masson F., Di Berardino-Besson W., et al. Homing phenotypes of tumor-specific CD8 T cells are predetermined at the tumor site by crosspresenting APCs. Immunity 2005; 22(2): 175–184, http://dx.doi.org/10.1016/j.immuni.2004.12.008.
- Okada H., Kohanbash G., Zhu X., et al. Immunotherapeutic approaches for glioma. Crit Rev Immunol 2009; 29(1): 1–42, http://dx.doi.org/10.1615/CritRevImmunol.v29.i1.10.
- Roy L.O., Poirier M.B., Fortin D. Transforming growth factor-beta and its implication in the malignancy of gliomas. Target Oncol 2014; http://dx.doi.org/10.1007/s11523-014-0308-y. [Epub ahead of print].
- Moore K.W., de Waal Malefyt R., Coffman R.L., et al. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol 2001; 19: 683–765, http://dx.doi.org/10.1146/annurev.immunol.19.1.683.
- Jiang J., Dingledine R. Role of prostaglandin receptor EP2 in the regulations of cancer cell proliferation, invasion, and inflammation. J Pharmacol Exp Ther 2013; 344(2): 360–367, http://dx.doi.org/10.1124/jpet.112.200444.
- Zhang J., Sarkar S., Cua R., et al. A dialog between glioma and microglia that promotes tumor invasiveness through the CCL2/CCR2/interleukin-6 axis. Carcinogenesis 2012; 33(2): 312–319, http://dx.doi.org/10.1093/carcin/bgr289.
- Shinohara H., Yagita H., Ikawa Y., Oyaizu N. Fas drives cell cycle progression in glioma cells via extracellular signal-regulated kinase activation. Cancer Res 2000; 60(6): 1766–1772.
- Salama A.K., Hodi F.S. Cytotoxic T-lymphocyte-associated antigen-4. Clin Cancer Res 2011; 17(14): 4622–4628, http://dx.doi.org/10.1158/1078-0432.ccr-10-2232.
- Gabrilovich D.I., Ostrand-Rosenberg S., Bronte V. Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol 2012; 12(4): 253–268. http://dx.doi.org/10.1038/nri3175.
- Sica A., Schioppa T., Mantovani A., et al., Tumour-associated macrophages are a distinct M2 polarised population promoting tumour progression: potential targets of anti-cancer therapy. Eur J Cancer 2006; 42(6): 717–727, http://dx.doi.org/10.1016/j.ejca.2006.01.003.
- Ohkura N., Kitagawa Y., Sakaguchi S. Development and maintenance of regulatory T cells. Immunity 2013; 38(3): 414–423, http://dx.doi.org/10.1016/j.immuni.2013.03.002.
- Mirghorbani M., Van Gool S., Rezaei N. Myeloid-derived suppressor cells in glioma. Expert Rev Neurother 2013; 13(12): 1395–1406, http://dx.doi.org/10.1586/14737175.2013.857603.
- Ooi Y.C., Tran P., Ung N., et al. The role of regulatory T-cells in glioma immunology. Clin Neurol Neurosurg 2014; 119: 125–132, http://dx.doi.org/10.1016/j.clineuro.2013.12.004.
- Grauer O.M., Nierkens S., Bennink E., et al. CD4+FoxP3+ regulatory T cells gradually accumulate in gliomas during tumor growth and efficiently suppress antiglioma immune responses in vivo. Int J Cancer 2007; 121(1): 95–105, http://dx.doi.org/10.1002/ijc.22607.
- Gousias K., Markou M., Arzoglou V., et al. Frequent abnormalities of the immune system in gliomas and correlation with the WHO grading system of malignancy. J Neuroimmunol 2010; 226(1–2): 136–142, http://dx.doi.org/10.1016/j.jneuroim.2010.05.027.
- Usatov S.A., Kovalenko A.P., Zallum Kh., et al. The immune status condition in patients with cerebral gliomas. Ukraїns’kiy medichniy al’manakh 2010; 13(1): 158–161.
- Brantley E.C., Benveniste E.N. Signal transducer and activator of transcription-3: a molecular hub for signaling pathways in gliomas. Mol Cancer Res 2008; 6(5): 675–684, http://dx.doi.org/10.1158/1541-7786.MCR-07-2180.
- Dix A.R., Brooks W.H., Roszman T.L., et al. Immune defects observed in patients with primary malignant brain tumors. J Neuroimmunol 1999; 100(1–2): 216–232, http://dx.doi.org/10.1016/S0165-5728(99)00203-9.
- Giometto B., Bozza F., Faresin F., et al. Immune infiltrates and cytokines in gliomas. Acta Neurochir (Wien) 1996; 138(1): 50–56.
- Lang R., Patel D., Morris J.J., et al. Shaping gene expression in activated and resting primary macrophages by IL-10. J Immunol 2002; 169(5): 2253–2263, http://dx.doi.org/10.4049/jimmunol.169.5.2253.
- Mancino A., Lawrence T. Nuclear factor-kappaB and tumor-associated macrophages. Clin Cancer Res 2010; 16(3): 784–789, http://dx.doi.org/10.1158/1078-0432.CCR-09-1015.
- Day E.D., Lassiter S., Woodhall B., et al. The localization of radioantibodies in human brain tumors. I. Preliminary exploration. Cancer Res 1965; 25(6): 773–778.
- Bidros D.S., Vogelbaum M.A. Novel drug delivery strategies in neuro-oncology. Neurotherapeutics 2009; 6(3): 539–546, http://dx.doi.org/10.1016/j.nurt.2009.04.004.
- Liu H.L., Hua M.Y., Chen P.Y., et al. Blood-brain barrier disruption with focused ultrasound enhances delivery of chemotherapeutic drugs for glioblastoma treatment. Radiology 2010; 255(2): 415–425, http://dx.doi.org/10.1148/radiol.10090699.
- Medyanik I.A., Mukhina I.V., Yakovleva E.I., et al. The method of temporary increase of blood-brain barrier penetration. Patent RF No.2391107. 2010.
- Bigner D.D., Brown M., Coleman R.E., et al. Phase I studies of treatment of malignant gliomas and neoplastic meningitis with 131I-radiolabeled monoclonal antibodies anti-tenascin 81C6 and anti-chondroitin proteoglycan sulfate Me1-14 F (ab’)2 — a preliminary report. J Neurooncol 1995; 24(1): 109–122.
- Riva P., Arista A., Franceschi G., et al. Local treatment of malignant gliomas by direct infusion of specific monoclonal antibodies labeled with 131I: comparison of the results obtained in recurrent and newly diagnosed tumors. Cancer Res 1995; 55(23 Suppl): 5952s–5956s.
- Eller J.L., Longo S.L., Hicklin D.J., Canute G.W. Activity of anti-epidermal growth factor receptor monoclonal antibody C225 against glioblastoma multiforme. Neurosurgery 2002; 51(4): 1005–1013; discussion 1013–1014.
- Neyns B., Sadones J., Joosens E., et al. Stratified phase II trial of cetuximab in patients with recurrent high-grade glioma. Ann Oncol 2009; 20(9): 1596–1603, http://dx.doi.org/10.1093/annonc/mdp032.
- Berezowska S., Schlegel J. Targeting ErbB receptors in high-grade glioma. Curr Pharm Des 2011; 17(23): 2468–2487, http://dx.doi.org/10.2174/138161211797249233.
- Vredenburgh J.J., Desjardins A., Herndon J.E. 2nd, et al. Phase II trial of bevacizumab and irinotecan in recurrent malignant glioma. Clin Cancer Res 2007; 13(4): 1253–1259, http://dx.doi.org/10.1158/1078-0432.CCR-06-2309.
- Friedman H.S., Prados M.D., Wen P.Y., et al. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol 2009; 27(28): 4733–4740, http://dx.doi.org/10.1200/JCO.2008.19.8721.
- Cecchi M., Vaiani M., Ceroti M., et al. A retrospective observational analysis to evaluate the off-label use of bevacizumab alone or with irinotecan in recurrent glioblastoma. Int J Clin Pharm 2013; 35(3): 483–487, http://dx.doi.org/10.1007/s11096-013-9765-0.
- Chamberlain M.C., Johnston S.K. Salvage therapy with single agent bevacizumab for recurrent glioblastoma. J Neurooncol 2010; 96(2): 259–269, http://dx.doi.org/10.1007/s11060-009-9957-6.
- Zhang G., Huang S., Wang Z. A meta-analysis of bevacizumab alone and in combination with irinotecan in the treatment of patients with recurrent glioblastoma multiforme. J Clin Neurosci 2012; 19(12): 1636–1640, http://dx.doi.org/10.1016/j.jocn.2011.12.028.
- Curtin J.F., Candolfi M., Fakhouri T.M., et al. Treg depletion inhibits efficacy of cancer immunotherapy: implications for clinical trials. PLoS One 2008; 3(4): e1983, http://dx.doi.org/10.1371/journal.pone.0001983.
- Mitchell D.A., Cui X., Schmittling R.J., et al. Monoclonal antibody blockade of IL-2 receptor alpha during lymphopenia selectively depletes regulatory T cells in mice and humans. Blood 2011; 118(11): 3003–3012, http://dx.doi.org/10.1182/blood-2011-02-334565.
- Blancher A., Roubinet F., Grancher A.S., et al. Local immunotherapy of recurrent glioblastoma multiforme by intracerebral perfusion of interleukin-2 and LAK cells. Eur Cytokine Netw 1993; 4(5): 331–341.
- Boiardi A., Silvani A., Ruffini P.A., et al. Loco-regional immunotherapy with recombinant interleukin-2 and adherent lymphokine-activated killer cells (A-LAK) in recurrent glioblastoma patients. Cancer Immunol Immunother 1994; 39(3): 193–197.
- Hayes R.L., Koslow M., Hiesiger E.M., et al. Improved long term survival after intracavitary interleukin-2 and lymphokine-activated killer cells for adults with recurrent malignant glioma. Cancer 1995; 76(5): 840–852.
- Dillman R.O., Duma C.M., Ellis R.A., et al. Intralesional lymphokine-activated killer cells as adjuvant therapy for primary glioblastoma. J Immunother 2009; 32(9): 914–919, http://dx.doi.org/10.1097/CJI.0b013e3181b2910f.
- Plautz G.E., Barnett G.H., Miller D.W., et al. Systemic T cell adoptive immunotherapy of malignant gliomas. J Neurosurg 1998; 89(1): 42–51, http://dx.doi.org/10.3171/jns.1998.89.1.0042.
- Saied A., Pillarisetty V.G., Katz S.C. Immunotherapy for solid tumors — a review for surgeons. J Surg Res 2014; 187(2): 525–535, http://dx.doi.org/10.1016/j.jss.2013.12.018.
- Yu J.S., Wheeler C.J., Zeltzer P.M., et al. Vaccination of malignant glioma patients with peptide-pulsed dendritic cells elicits systemic cytotoxicity and intracranial T-cell infiltration. Cancer Res 2001; 61(3): 842–847.
- Kikuchi T., Akasaki Y., Irie M., et al. Results of a phase I clinical trial of vaccination of glioma patients with fusions of dendritic and glioma cells. Cancer Immunol Immunother 2001; 50(7): 337–344.
- Ardon H., Van Gool S.W., Verschuere T., et al. Integration of autologous dendritic cell-based immunotherapy in the standard of care treatment for patients with newly diagnosed glioblastoma: results of the HGG-2006 phase I/II trial. Cancer Immunol Immunother 2012; 61(11): 2033–2044, http://dx.doi.org/10.1007/s00262-012-1261-1.
- Kozlov V.A., Chernykh E.R. Current immune therapy problems in oncology. Byulleten’ SO RAMN 2004; 2(112): 13–19.
- Rosenberg S.A., Yang J.C., Restifo N.P. Cancer immunotherapy: moving beyond current vaccines. Nat Med 2004; 10(9): 909–15, http://dx.doi.org/10.1038/nm1100.
- Sampson J.H., Archer G.E., Mitchell D.A., et al. Tumor-specific immunotherapy targeting the EGFRvIII mutation in patients with malignant glioma. Semin Immunol 2008; 20(5): 267–275, http://dx.doi.org/10.1016/j.smim.2008.04.001.
- Sampson J.H., Heimberger A.B., Archer G.E., et al. Immunologic escape after prolonged progression-free survival with epidermal growth factor receptor variant III peptide vaccination in patients with newly diagnosed glioblastoma. J Clin Oncol 2010; 28(31): 4722–4729, http://dx.doi.org/10.1200/JCO.2010.28.6963.
- Yajima N., Yamanaka R., Mine T., et al. Immunologic evaluation of personalized peptide vaccination for patients with advanced malignant glioma. Clin Cancer Res 2005; 11(16): 5900–5911, http://dx.doi.org/10.1158/1078-0432.CCR-05-0559.
- Mine T., Sato Y., Noguchi M., et al. Humoral responses to peptides correlate with overall survival in advanced cancer patients vaccinated with peptides based on pre-existing, peptide-specific cellular responses. Clin Cancer Res 2004; 10(3): 929–937, http://dx.doi.org/10.1158/1078-0432.CCR-1117-3.
- Terasaki M., Shibui S., Narita Y., et al. Phase I trial of a personalized peptide vaccine for patients positive for human leukocyte antigen — A24 with recurrent or progressive glioblastoma multiforme. J Clin Oncol 2011; 29(3): 337–44, http://dx.doi.org/10.1200/JCO.2010.29.7499.
- Sampetrean O., Saya H. Characteristics of glioma stem cells. Brain Tumor Pathol 2013; 30(4): 209–214, http://dx.doi.org/10.1007/s10014-013-0141-5.
- Heywood R.M., Marcus H.J., Ryan D.J., et al. A review of the role of stem cells in the development and treatment of glioma. Acta Neurochir (Wien) 2012; 154(6): 951–969; discussion 969, http://dx.doi.org/10.1007/s00701-012-1338-9.
- Qiu B., Zhang D., Tao J., et al. Human brain glioma stem cells are more invasive than their differentiated progeny cells in vitro. J Clin Neurosci 2012; 19(1): 130–134, http://dx.doi.org/10.1016/j.jocn.2011.06.014.
- Singh S.K., Hawkins C., Clarke I.D., et al. Identification of human brain tumour initiating cells. Nature 2004; 432(7015): 396–401, http://dx.doi.org/10.1038/nature03128.
- Dean M., Fojo T., Bates S. Tumour stem cells and drug resistance. Nat Rev Cancer 2005; 5(4): 275–284, http://dx.doi.org/10.1038/nrc1590.
- Persano L., Rampazzo E., Basso G., et al. Glioblastoma cancer stem cells: role of the microenvironment and therapeutic targeting. Biochem Pharmacol 2013; 85(5): 612–622, http://dx.doi.org/10.1016/j.bcp.2012.10.001.
- Bao S., Wu Q., McLendon R.E., et al. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature 2006; 444(7120): 756–760, http://dx.doi.org/10.1038/nature05236.
- Huang Z., Cheng L., Guryanova O.A., et al. Cancer stem cells in glioblastoma — molecular signaling and therapeutic targeting. Protein Cell 2010; 1(7): 638–655, http://dx.doi.org/10.1007/s13238-010-0078-y.
- Tate M.C., Aghi M.K. Biology of angiogenesis and invasion in glioma. Neurotherapeutics 2009; 6(3): 447–457, http://dx.doi.org/10.1016/j.nurt.2009.04.001.
- Hovinga K.E., Stalpers L.J., van Bree C., et al. Radiation-enhanced vascular endothelial growth factor (VEGF) secretion in glioblastoma multiforme cell lines — a clue to radioresistance? J Neurooncol 2005; 74(2): 99–103, http://dx.doi.org/10.1007/s11060-004-4204-7.
- Ueda R., Iizuka Y., Yoshida K., et al. Identification of a human glioma antigen, SOX6, recognized by patients' sera. Oncogene 2004; 23(7): 1420–1427, http://dx.doi.org/10.1038/sj.onc.1207252.
- Ueda R., Ohkusu-Tsukada K., Fusaki N., et al. Identification of HLA-A2- and A24-restricted T-cell epitopes derived from SOX6 expressed in glioma stem cells for immunotherapy. Int J Cancer 2010; 126(4): 919–929, http://dx.doi.org/10.1002/ijc.24851.
- Pellegatta S., Poliani P.L., Corno D., et al. Neurospheres enriched in cancer stem-like cells are highly effective in eliciting a dendritic cell-mediated immune response against malignant gliomas. Cancer Res 2006; 66(21): 10247–10252, http://dx.doi.org/10.1158/0008-5472.CAN-06-2048.
- Xu Q., Liu G., Yuan X., et al. Antigen-specific T-cell response from dendritic cell vaccination using cancer stem-like cell-associated antigens. Stem Cells 2009; 27(8): 1734–1740, http://dx.doi.org/10.1002/stem.102.










