Study of the Allergizing Properties of Nanocolloid,99mTc–Al2O3 Radiopharmaceutical in Experiment
The aim of the investigation was to assess the allergizing properties of a new nanocolloidal preparation based on aluminum gamma-oxide labeled by technetium-99m for scintigraphic and intraoperative detection of sentinel lymphatic nodes in experiment.
Materials and Methods. A series of experiments was performed on 80 guinea pigs, 30 Balb/c mice, 40 CBA/CaLac mice and 70 outbred mice of both genders. To study allergenicity various doses of preparation were administered subcutaneously, intramuscularly, intravenously, intradermally as a single injection or by a course. Allergizing properties of the radiopharmaceutical were investigated according to the “Guidelines on the assessment of allergizing properties of drugs”.
Results. Administration of Nanocolloid,99mTc‒Al2O3 radiopharmaceutical did not result in the intensification of anaphylaxis systemic reaction in the animals of the examined groups relative to the control. A single injection did not cause inflammatory reaction to Concanavalin A in the examined animals. When guinea pigs were immunized by the preparation, no delayed allergic reaction was revealed. Studying sensitizing effect of the preparation by 20 skin applications no redness or edema of the skin at the site of application was observed.
Conclusion. Nanocolloid,99mTc‒Al2O3 radiopharmaceutical in the studied doses and under the selected experimental conditions does not possess allergizing properties.
Sentinel lymphatic nodes are the nodes a malignant tumor reaches first. If they are not affected by a metastatic process, all the rest regional lymphatic nodes are supposed to remain intact [1, 2]. A wide implementation of the technologies detecting such nodes will improve the life quality of a large number of sick people [1‒5]. An optimal method of detecting these nodes is an application of technetium-99m labeled colloid materials for scintigraphic or radiometric determination of the node localization with the following express-histological examination [6, 7].
It should be noted, that a short lifetime technetium-99m is the most common radionuclide for diagnostic investigations in all fields of medicine. Radiopharmaceuticals (RP) based on it are used in more than 87% of all radionuclide investigations aimed at the detection of various diseases and assessment of the living system state. This is mainly owing to its nuclear-physical characteristics: a relatively short half-time of T1/2 (6.02 h) and g-radiation energy (0.1405 MeV), providing a small exposure dose and, at the same time, a sufficient penetration power for radiometric measurements. Moreover, a rich coordinating chemistry of technetium makes it possible to obtain on its basis various simple and complex biologically active compounds with the given properties [8‒10]. At present, technetium is the most common radionuclide in nuclear medicine, as it can be produced directly in medical settings from 99Mo/99mTc generators.
The main reasons for using aluminum oxide as a carrier of technetium-99m label are its low toxicity in combination with good adsorption properties, availability and a low price.
Nanocoloid,99mTc‒Al2O3 radiopharmaceutical is a new preparation developed in National Research Tomsk Polytechnic University (Russia) for diagnosis of sentinel lymphatic nodes in oncologic patients.
The aim of the investigation was to assess the allergizing properties of a new nanocolloidal preparation based on aluminum gamma-oxide labeled by technetium-99m for visualization of sentinel lymphatic nodes.
Materials and Methods. 80 guinea pigs of both genders 6‒8 weeks of age weighing 250‒300 g, 30 Balb/c mice, 40 CBA/CaLac mice and 70 outbred mice of both genders aging 6‒8 weeks and weighing 18‒22 g were used in the experiment.
Experimental animal care and investigations were performed in accordance with ethical principles established by European Convention for the Protection of Vertebrata used for Experimental and other Scientific Purposes (the Convention was passed in Strasburg, March, 18, 1986, adopted in Strasburg, June, 15, 2006).
A therapeutic dose (TD) of RP amounted to 0.09 ml/kg for mice and 0.04 ml/kg for guinea pigs. The doses used in the experiment corresponded to those introduced to a man, conversion of doses for animals was done according to Freireich.
To study allergenicity various doses of preparation were administered subcutaneously, intramuscularly, intravenously, intradermally as a single injection or in a course. A solvent (isotonic solution of sodium chloride) was introduced in the equivalent dose to the animals of the control group of the appropriate gender and age.
Allergizing properties of the radiopharmaceutical were investigated according to the “Guidelines on the assessment of allergizing properties of drugs” [11].
The following techniques were used to assess the allergizing properties of the preparation:
1. Reaction of generalized anaphylaxis (anaphylactic shock) in guinea pigs. The animals used in the experiment (n=30, 15 males and 15 females) were mature conventional guinea pigs. The mass of males was 270‒300 g, and that of females — 250‒280 g, age 8 weeks. Sensitization was performed by introducing the examined preparation three times parenterally: the first injection was given subcutaneously, the next two intramuscularly on alternate days in 1 and 10 TD (n=20). 2 ml/kg of isotonic solution of sodium chloride was given to the animals of the control group (n=10) according to the same scheme. Anaphylactogenic properties of the preparation were determined by its administration on day 14 after the last sensitizing application. The testing intravenous introduction of the examined preparation was performed in the total sensitizing dose, i.e. 0.12 and 1.2 ml/kg. Nonsesitized guinea pigs, which were injected intravenously in the dose 0.12 ml/kg, served as a control. Anaphylactic reaction intensity was measured as suggested by Weigle.
2. Conjunctival test on guinea pigs. Preliminary sensitizing by subcutaneous introduction of RP in the dose of 0.04 and 0.4 ml/kg during 5 days was conducted to the animals of the experimental group (n=20). The test was performed in the following way: 20 μl 0.01% solution of RP (the dose, causing no irritation in the intact animals) was dropped into the left eye of each animal, while isotonic solution of sodium chloride in the same volume was dropped into the right eye. Eye conjunctiva condition was evaluated 15 min and 24‒48 h after the test. The control group consisted of 10 intact guinea pigs.
3. Delayed hypersensitivity reaction on mice. Autbred mice (n=20) were sensitized once by subcutaneous introduction to the tail basis of 60 μl of emulsion of 10 mM RP in Freund’s complete adjuvant in the ratio 1:1. Hanks solution was used to prepare the emulsion. Control animals (n=20) were sensitized by the emulsion in Freund’s adjuvant with Hanks solution in the same way as the experimental animals.
To reveal sensitization 40 μl 10 mM of RP in Hanks solution were injected to the pad of the mice hind leg 5 days later. 6 and 24 h after the injection delayed hypersensitivity reaction intensity was estimated by the reaction index (RI), which was calculated individually for each animal by a formula:

where Wex is “experimental” leg mass; Wc is “control” leg mass.
4. Active skin anaphylaxis on mice. Bulb/c mice (n=20) were sensitized by introducing RP three times parenterally: the first injection subcutaneously, the following two intramuscularly on alternate days in the volume of 1 and 10 TD. Permitted RP injection was performed 14 days after the last sensitizing administration in twofold dilutions of 0.01% preparation solution intradermally — 15 μl (increasing the volume of isotonic solution of sodium chloride up to 30 μl) and 30 μl. 20 min later 0.5 ml 1% Evans blue solution was injected intravenously to the mice. In 30 min animals were withdrawn from the experiment in CO2-chamber, and the size of the blue spot on the internal side of the skin at the site of injection was determined. Permitted to use RP injections and Evans blue were also given to the control animals (n=10), which were introduced only the solvent (physiological salt solution) on the days of sensitizing the experimental animals.
5. Indirect reaction of mast cell degranulation. After completing a 5-day course of introducing the preparation to the CBA/CaLac mice (n=40) in 1 and 10 TD, the animals were decapitated in the CO2-chamber and blood serum was obtained, which was then tested for the ability to induce mast cell degranulation in the presence of a specific antigen (the preparation under study). To obtain mast cells, 4‒5 ml of Hanks solution without phenol red was injected intraperitoneally to the intact animals after decapitation, and following a slight massage of the abdominal wall exudate was collected to the siliconized tube for 1‒1.5 min. Specimens were prepared on the degreased glass slide, stained by 0.3% alcohol solution of neutral red and dried at a room temperature. On one side of the slide 0.03 ml of the mast cell suspension, 0.03 ml of the examined animal serum and 0.03 ml of 0,01% preparation solution were mixed (experiment), on the other side there was a mixture of 0.03 ml of the mast cell suspension, 0.03 ml of the examined animal serum and 0.03 ml of Hanks solution (control). Then the specimens were covered with cover glasses and incubated for 15 min in a thermostat at 37°C. Specimens were examined under a microscope with ×20 magnification.
The results were assessed using a differential way of calculation, mast cell degranulation index (MCDI) was found by a formula:

where a, b, c, d, mean the number of degranulated cells corresponding to the degree of granulation (weak, moderate, sharp and fully degranulated cells).
6. Reaction of inflammation using Concavalin A. The experiments were carried out on outbred mice (n=20) weighing 18‒22 g. RP was introduced subcutaneously in two volumes of 1 and 10 TD only once. 10 ml/kg of isotonic solution of sodium chloride was similarly introduced to the mice of the control group (n=10). Concavalin A in the dose of 100 μg/20 g of body mass was injected subplantarly and the same volume of isotonic solution of sodium chloride was injected to the contralateral extremity of the mice from the experimental and control groups 3 h after RP. An hour later the mice were decapitated, the mass of legs was determined, and RI of inflammation calculated by a formula:
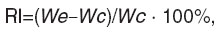
where Wex is the mass of the hind leg foot, into the pad of which Concavalin A was injected; Wc is the mass of the hind leg foot, into the pad of which isotonic solution of sodium chloride was injected.
7. Method of skin applications on guinea pigs. Investigation of sensitizing effect of RP was conducted by 20 repeated skin applications 2×2 cm in size 5 times a week on the lateral surface of the guinea pig bodies (n=10). Three drops of 0.01% RP were applied. The first test was performed after 10 applications, the second one after 20. 10 intact guinea pigs composed a control group.
The data obtained were processed by the method of variational statistics. Arithmetic mean (X) and error of the arithmetic mean (m) were counted for each sample. Testing for normal distribution was done using Shapiro–Wilk criterion. Comparison of the sample means was performed by Student’s t-criterion in case of normal distribution or by Kruskal–Wallis criterion for k-independent samples (k>2) in case the distribution differed from normal.
Results and Discussion
Reaction of generalized anaphylaxis (anaphylactic shock). Testing intravenous introduction of RP did not result in anaphylactic reaction in guinea pigs. Anaphylaxis index both in the control and examined animal groups of both genders was less than 1.0 (Table 1).
|
|
Conjunctival test. Conjunctival test revealed no signs of conjunctiva or lacrimal ducts reddening in the eyes of male or female guinea pigs, speaking of the absence of hypersensitivity to the preparation.
Delayed hypersensitivity reaction in mice. Statistically significant differences of delayed hypersensitivity reaction index in the examined groups, receiving RP with Freund’s adjuvant, in male or female mice was not observed 6 and 24 h after the test compared to the control group (Table 2).
 Table 2. Effect of the radiopharmaceutical (RP) injected with Freund’s adjuvant on the intensity of the delayed hypersensitivity reaction in outbred mice ( Table 2. Effect of the radiopharmaceutical (RP) injected with Freund’s adjuvant on the intensity of the delayed hypersensitivity reaction in outbred mice ( ±m) ±m)
|
Active skin anaphylaxis on mice. After intradermal permitted injection of 0.01% RP solution to the Bulb/c mice the diameter of the stained spot did not significantly differ either in males or in females in the experimental groups using various doses of the preparation in comparison with the respective values in the control groups (Table 3).
|
|
Indirect reaction of mast cell degranulation. Serum obtained from CBA/CaLac mice of both genders, to which RP was introduced by a course, did not induce statistically significant changes of the MCDI in comparison with the control group both in the presence and absence of the examined preparation (Table 4).
 Table 4. Effect of the course introduction of the radiopharmaceutical (RP) in the doses of 0.09 and 0.9 ml/kg on the reaction of mast cell degranulation in CBA/CaLac mice ( Table 4. Effect of the course introduction of the radiopharmaceutical (RP) in the doses of 0.09 and 0.9 ml/kg on the reaction of mast cell degranulation in CBA/CaLac mice ( ±m) ±m)
|
Inflammatory reaction to Concavalin A in mice. Introduction of RP to outbred mice of both genders did not affect the index of the inflammatory reaction to Concavalin A (Table 5).
 Table 5. Effect of a single introduction of the radiopharmaceutical (RP) in the doses of 0.09 and 0.9 ml/kg on the index of the inflammatory reaction to Concavalin A in the outbred mice ( Table 5. Effect of a single introduction of the radiopharmaceutical (RP) in the doses of 0.09 and 0.9 ml/kg on the index of the inflammatory reaction to Concavalin A in the outbred mice ( ±m) ±m)
|
Method of skin applications. When studying a sensitizing effect of RP by skin applications neither reddening nor skin edema were noted at the site of preparation application both after 10 and 20 applications, skin reaction in the examined groups (males and females) did not differ from the control.
Thus, studying the allergizing properties of Nanocolloid,99mTc‒Al2O3 RP in the experiments on various animals no statistically significant differences of the results in the control and examined groups in males and females were found. The preparation under study does not induce generalized anaphylaxis (anaphylactic shock), does not influence active skin anaphylaxis, pseudoallergic inflammatory reaction on Concavalin A in mice, and indirect reaction of mast cell degranulation in mice. The preparation injected with Freund’s adjuvant does not intensify inflammation in delayed hypersensitivity reaction. Hypersensitivity to the examined preparation is absent in all experimental animals.
Conclusion. Nanocolloid,99mTc‒Al2O3 radiopharmaceutical in the studied doses and under the selected experimental conditions does not possess allergizing properties.
Study Funding. The work was supported by the Federal target program “PHARMA-2020” (2011–2014) “Preclinical investigations of a novel lymphotropic radiopharmaceutical preparation based on aluminum gamma-oxide labeled by technetium-99m”.
Conflicts of Interest. The authors have no conflicts of interest.
References
- Chernov V.I., Sinilkin I.G., Shiryaev S.V. Radionuklidnoe vyyavlenie storozhevykh limfaticheskikh uzlov. V kn.: Natsional’noe rukovodstvo po radionuklidnoy diagnostike [Radionuclide detection of sentinel lymphatic nodes. In: National guideline on radionuclide diagnosis]. Pod red. Lishmanova Yu.B., Chernova V.I. [Lishmanov Yu.B., Chernov V.I. (editors)]. Tomsk: STT; 2010; p. 336–343.
- Paredes P., Vidal-Sicart S., Zanуn G., Pahisa J., Fernández P.L., Velasco M., Santamaría G., Ortín J., Duch J., Pons F. Clinical relevance of sentinel lymph node in the internal mammary chain in breast cancer patients. Eur J Nucl Med Mol Imaging 2005; 32(11): 1283–1287, http://dx.doi.org/10.1007/s00259-005-1867-z.
- Kanayev S.V., Novikov S.N., Zhukova L.A., Zotova O.V., Semiglazov V.F., Krivorotko P.V. Lymphoscintigraphy of individual lymph flow for radiotherapy planning in breast cancer. Voprosy onkologii 2011; 57(5): 616–621.
- Chernov V.I., Afanasyev S.G., Sinilkin I.G., Titskaya A.A., Avgustinovich A.V. Radionuclide diagnosis for detection of sentinel lymph nodes. Sibirskiy onkologicheskiy zhurnal 2008; 4: 5–10.
- Schauer A.J., Becker W., Reiser M., Possinger K. The sentinel lymph node concept. Springer Verlag, Heidelberg Berlin New York; 2005, http://dx.doi.org/10.1007/b137529.
- Afanasyev S.G., Avgustinovich A.V., Chernov V.I., Sinilkin I.G. Radio-guided sentinel lymph node detection in gastric cancer patients. Sibirskiy onkologicheskiy zhurnal 2009; 34(4): 27–32.
- Maza S., Valencia R., Geworski L., Zander A., Guski H., Winzer K.J., Munz D.L. Peritumoural versus subareolar administration of technetium-99m nanocolloid for sentinel lymph node detection in breast cancer: preliminary results of a prospective intra-individual comparative study. Eur J Nucl Med Mol Imaging 2003; 30(11): 651–688, http://dx.doi.org/10.1007/s00259-003-1128-y.
- Jurisson S.S., Lydon J.D. Potential technetium small molecule radiopharmaceuticals. Chem Rev 1999; 99(9): 2205–2218, http://dx.doi.org/10.1021/cr980435t.
- Baishya R., Nayak D.K., Karmakar S., Chattopadhyay S., Sachdeva S.S., Sarkar B.R., Ganguly S., Debnath M.C. Synthesis and evaluation of technetium-99m-labeled bioreductive pharmacophores conjugated with amino acids and peptides for tumor imaging. Chem Biol Drug Des 2015; 85(4): 504–517, http://dx.doi.org/10.1111/cbdd.12437.
- Geskovski N., Kuzmanovska S., Simonoska Crcarevska M., Calis S., Dimchevska S., Petrusevska M., Zdravkovski P., Goracinova K. Comparative biodistribution studies of technetium-99 m radiolabeled amphiphilic nanoparticles using three different reducing agents during the labeling procedure. J Labelled Comp Radiopharm 2013; 56(14): 689–695, http://dx.doi.org/10.1002/jlcr.3097.
- Rukovodstvo po provedeniyu doklinicheskikh issledovaniy lekarstvennykh sredstv. Chast’ I [Guideline for preclinical studies drugs. Part I]. Moscow: Grif i K; 2012; 944 p.

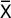 ±m)
±m)










