Physicochemical Properties of Intravitreal Implant Based on Chitosan/Polyvinyl Alcohol Saturated with 5-Fluorouracil
The aim of the investigation was to evaluate the possibility of using intravitreal introduction of the implant based on chitosan-polymer film saturated with 5-fluorouracil.
Materials and Methods. There was performed a study of physicochemical properties of chitosan/polyvinyl alcohol-based implant saturated with 5-fluorouracil (5-FU) in concentrations of 0.05 and 0.1 ml. The implant size was 8.0×1.0×0.35 mm. The process of drug release was studied by UV spectroscopy based on the characteristic absorption maximum. To assess the sterilization ability of chitosan films, their thermal stability was studied. The possibilities of interaction between chemical groups of chitosan, polyvinyl alcohol and 5-FU were studied by analyzing the infrared spectra of these substances.
Results. It was established that the process of drug release from the system occurs in three stages: 1) sorption of water by the film and swelling; 2) the drug diffusing in the film to the interphase boundary between the polymer system and the environment; 3) the drug diffusing into the solvent volume. Release of 5-FU from the implant to Ringer–Locke solution occurs almost completely within 7–8 h without undergoing any change. Thermal degradation of the implant begins at the temperature of 200°C. Infrared spectroscopy data evidence that 5-FU immobilized on chitosan film with polyvinyl alcohol undergoes no chemical changes and, consequently, does not lose its pharmacological properties.
Conclusion. The study of physicochemical properties of chitosan/polyvinyl alcohol-based implant saturated with 5-FU cytostatic proves the feasibility of its use in ophthalmology to reduce the initial peak concentration of 5-FU and, consequently, eliminate its toxic effects and prolong the drug action.
Proliferative vitreoretinopathy (PVR) is one of the most severe eye diseases and a serious medical and social problem. PVR is considered to be a typical pathological process within the eye characterized by local scarring as means of eliminating tissue alteration occurring during such an ophthalmic diseases as retinal detachment, hemophthalmia, trauma, diabetes [1].
In vision disability, PVR accounts for 2–9% and 84–89% of those suffering from the disease are persons of working age [2–4].
Proliferative vitreoretinopathy requires complex surgical treatment which is often performed in several stages [5]. If the disease remains untreated, blindness occurs in 100% of cases [6–8].
In recent years, progress has been made in retinal detachment surgery, which allowed reducing the number of intra- and postoperative complications, significantly improving anatomical and functional results of operations for this pathology. However, despite the new level of modern diagnostic possibilities and a significant step forward in the field in vitreoretinal surgery, the number of successful operations, according to many authors, is only 61.5 to 97.5% [9–13].
PVR progression in the postoperative period is one of the main causes of unsuccessful surgical treatment for retinal detachment and it is observed in 2.2–29.4% of cases [9, 11, 13, 14]. Retinal detachment recurrence due to PVR progression occurs in 2.20 to 20.0% of cases [11, 13, 15–17]. Therefore, the search for and development of new treatments reducing surgery invasiveness and the risk of operative and postoperative complications is under way.
At present, there have been new trends in PVR treatment such as application of antiproliferative agents on various carrier implants used during intravitreal interventions. In recent years there has been a growing interest of specialists for applying as a carrier chitosan polymer material which is completely destroyed and absorbed by the body, has anti-inflammatory and antiproliferative effect, high biocompatibility, improves regeneration of cells and tissues [18–20]. Kazakh Scientific Research Institute of Eye Diseases in cooperation with the laboratory of polymer synthesis, A.B. Bekturov Institute of Chemical Sciences (Kazakhstan), developed the vitreosyneretic Vitrenal which is an aqueous solution of chitosan polymer [6]. Clinical studies have confirmed the efficacy of intravitreal introduction of Vitrenal in surgical treatment for PVR with retinal detachment and traumatic eye injuries.
Currently, one of the trends in vitreoretinal surgery is the use of medicines whose action is aimed at inhibiting scar tissue formation, in particular, cytostatics. The most famous representative of this group is 5-fluorouracil (5-FU) [21, 22]. This therapeutic agent is an antimetabolite inhibiting DNA synthesis and proliferation of fibroblasts. However, different authors show ambiguous results of its application, which determines the necessity of further investigations to explore the drug potential, in particular, as chitosan implant coating.
The aim of the investigation was to evaluate the possibility of using intravitreal introduction of the implant based on chitosan-polymer film saturated with 5-fluorouracil.
Materials and Methods. There was performed a study of physicochemical properties of chitosan and polyvinyl alcohol based implant saturated with 5-fluorouracil (5-FU) in concentrations of 0.05 (250 mg/g) and 0.1 ml (500 mg/g), which corresponds to 2.5 and 5.0 mg of dry matter. Polyvinyl alcohol was used to improve physicochemical properties of the films. The dosage of 5-FU was chosen for a good reason. According to the literature, 0.1–0.15 ml of 5% 5-FU is considered the optimal therapeutic dose. In the study by D.N. Sharipova [21], marked toxic changes in the eye structures were observed when a single intravitreal injection of 0.15 ml of 5-FU was administered, whereas introduction of the same dose on polyurethane carrier had no such effect. We chose the therapeutic dose (0.1 ml) and the dose 2 times lower than the therapeutic one (0.05 ml) in recognition of the fact that the implant (chitosan) by itself provides antiproliferative and anti-inflammatory action.
Scanning electron microscopy images of polymeric medicinal forms were obtained using the Superprobe-733 electron probe microanalyzer (JEOL, Japan) equipped with energy dispersive spectrometer INCA Energy (Oxford Instruments, USA). The powder was applied to the conductive tape, then, to improve image contrast, it was covered with a thin layer of gold using the Fine Coat installation (JEOL, Japan). Imaging was carried out in the mode of secondary electrons.
To determine the kinetics of 5-FU release from the chitosan film, a special device consisting of a metal basket, a thermostatic glass and a mechanical stirrer was used. The drug release was studied in vitro. For this, a certain quantity of chitosan films was placed in a metal basket immersed in 70 ml of water at room temperature. Constant rate of release medium stirring (100 rpm) was provided with a magnetic stirrer, temperature control was maintained using a flow cell. At certain time intervals, 2 ml of solution was taken to determine the drug content by UV spectroscopy.
To assess the ability of the implants for sterilization, their thermal stability was studied by thermogravimetric analysis using the TGA/SDTA 851 device (Mettler Toledo, Switzerland).
To evaluate the possibility of chemical interaction between chitosan, polyvinyl alcohol, and 5-FU, infrared spectra of samples of these substances were recorded and analyzed. IR spectra were recorded on the Nicolet 5700 FT-IR spectrophotometer (Thermo Electron, USA) with Fourier transform in the region of 4,000–400 cm–1. The samples were prepared in the form of tablets with КВr crystals.
Results and Discussion
Scanning electron microscopy. Scanning electron microscopy of chitosan and the implant was performed using the Superprobe 733 electron probe microanalyzer equipped with energy dispersive spectrometer INCA Energy (Figure 1). The smooth surface of chitosan was clearly visible, the implant had a random distribution of large structures with pores of the same size, coming up to 42.68 nm. Thus, the implant had mesopores.

|
Figure 1. Scanning electron microscopy of chitosan (a) and 5-fluorouracil-saturated implant (b) |
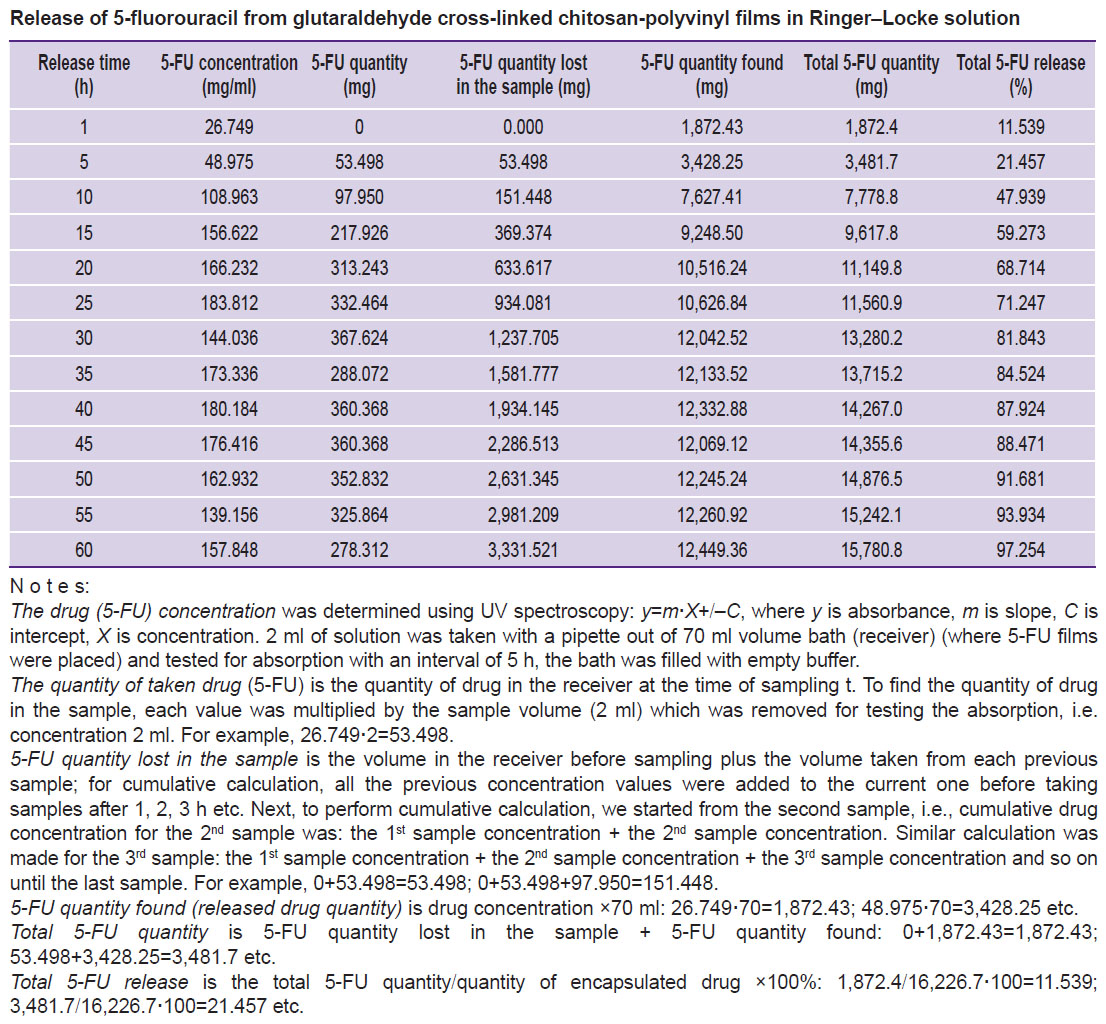
The release of 5-FU from the implant. To assess the prolonged release properties, 5-FU release from chitosan-polyvinyl films cross-linked with glutaraldehyde was studied in vitro. The release process was studied by UV spectroscopy based on the characteristic absorption maximum of the drug at λ=266 nm. The spectra were detected on the Jasco UV/VIS 7850 spectrophotometer (JASCO International, Japan) in quartz cuvettes of 10 mm thickness at 25°C. The amount of 5-FU was identified with the calibration graph of optical density versus concentration. Freshly prepared Ringer–Locke solution was used as the environment for release.
It was established that the process of drug release from the system consists of three stages: 1) sorption of water by the implant and its swelling; 2) the drug diffusing in the implant to the interphase boundary between the polymer system and the environment; 3) the drug diffusing into the solvent volume.
Chitosan films with 250 and 500 mg/g of 5-FU were submerged into the solution to determine the effect of the drug dose (concentration) on release kinetics. It was noted that the change in the drug concentration in the polymer matrix had no significant effect on the diffusion process. 5-FU diffused almost completely in Ringer–Locke solution within 60–70 h (Figure 2). The obtained parameters of the release are presented in the Table.

|
Figure 2. Release of 5-fluorouracil from chitosan-polyvinyl film cross-linked by glutaraldehyde at the drug concentrations of 250 mg/g (1) and 500 mg/g (2) |
| Release of 5-fluorouracil from glutaraldehyde cross-linked chitosan-polyvinyl films in Ringer–Locke solution |
Kinetic curve profile suggests that the drug release takes place according to first-order kinetics and is controlled by diffusion of the therapeutic agent in the matrix.
It is known that with the implant thickness increasing 3 times the drug diffusion coefficient decreases approximately 2 times. Diffusion of the therapeutic agent in the matrix plays the limiting role in the process of 5-FU release from the chitosan implant as evidenced by the inverse relationship between release rate and film thickness.
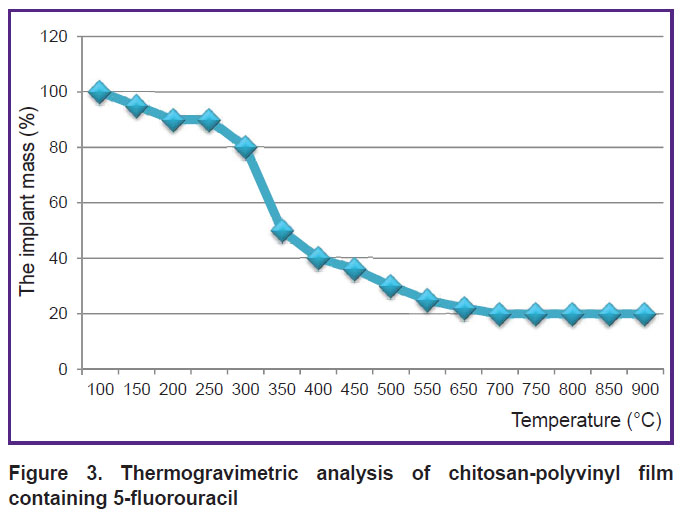
Evaluating the thermal stability of the chitosan implant saturated with 5-FU. Thermal properties of the chitosan implant were studied using thermogravimetric analysis on the TGA/SDTA 851 device. Thermogravimetry is a method to determine mass variation when the substance undergoes controlled heat treatment. The analysis was carried out in the temperature range from 50 to 900°C with heating rate 5°C/min (Figure 3).
| Figure 3. Thermogravimetric analysis of chitosan-polyvinyl film containing 5-fluorouracil |
Thermogravimetric analysis curve for the chitosan implant with 5-FU shows that thermal degradation of the implant begins at the temperature of 250°C. In general, the degradation process occurs in the temperature range of 250–500°C.
The obtained data indicate that 5-FU-saturated implant based on chitosan and polyvinyl alcohol can be sterilized in an autoclave.
The study of interaction between chemical groups of chitosan, polyvinyl alcohol, and 5-FU. To assess the level of interaction between chemical groups of glutaraldehyde cross-linked chitosan, polyvinyl alcohol, and 5-FU, infrared spectra of samples of these substances were recorded on the Nicolet 5700 FT-IR spectrophotometer with Fourier transform in the region of 4,000–400 cm–1 and analyzed. The samples were prepared in the form of tablets with КВr crystals.
Figure 4 shows the infrared spectra of 5-FU, cross-linked chitosan films containing 5-FU, and cross-linked chitosan-polyvinyl film containing 5-FU, respectively. The main 5-FU peaks are observed in the spectrum regions of 3,084 cm–1 (N–H), 1,689 cm–1 (C=O), 1,250 cm–1 (C=C) and 1,180 cm–1 (C–F) (Figure 4 (a)).

|
Figure 4. Infrared spectra of 5-FU (a), glutaraldehyde cross-linked chitosan film containing 5-FU (b) and cross-linked chitosan-polyvinyl film containing 5-FU (c) |
Absorbance in 5-FU-containing chitosan film at 3,127 cm–1 demonstrated peaks of O–H chitosan groups and N–H stretching vibrations of 5-FU that overlapped each other. C=O-vibrations at 1,683 cm–1 were detected in chitosan film spectrum, which also indicates the presence of overlapping.
Absorption bands 1,247 cm–1 (C=C) and 1,172 cm–1 (C–F bond) showed 5-FU presence in the film spectrum (Figure 4 (b)). The presence of azomethine group in chitosan-glutaraldehyde interaction product was identified by the peak of C=N stretching vibrations, which was easily detected in the spectra at 1,634 cm–1 frequency due to high extinction coefficient. This peak intensity increased with the increasing number of protonated amino-groups in chitosan.
There was observed a transition of NH2− groups to –N=C− groups, disappearance of OH− groups and formation of acetal and simple ether groups in the reaction of OH− groups with CH2O.
Infrared spectra of chitosan-polyvinyl film containing 5-FU are shown in Figure 4 (c). Absorption in the area of 3,134 cm–1 indicates the presence of O–H group of chitosan, O–N group of polyvinyl alcohol and N–H bond of 5-FU. In the area of 1,664 cm–1 there appear absorption bands caused by C=O bond participation in the skeletal vibrations of polyvinyl alcohol molecules in chitosan-polyvinyl film.
Figure 4 also shows glutaraldehyde effect on the chemical structure of chitosan-polyvinyl film. Decrease of OH- and C–O bonds of polyvinyl alcohol was revealed in the region of 1,090 cm–1, which indicates the appearance of –C–O–C group at 1,090 cm–1.
The obtained data show that 5-FU and polymers are compatible. 5-FU on chitosan with polyvinyl alcohol undergo no chemical changes and, consequently, loses no pharmacological properties.
Conclusion. The use of an intravitreal implant based on natural polymer chitosan saturated with 5-fluorouracil cytostatic provides gradual release of 5-fluorouracil during 60–70 h, which reduces primary peak concentration of the drug and prevents exceeding its therapeutic concentration in the environment. The established physicochemical properties of 5-FU-immobilized chitosan implant speak of the possibility to use it in ophthalmology with the aim of prolonging the drug action. Well-known antiproliferative and anti-inflammatory properties of natural polysaccharide chitosan confirm the feasibility of its use as a medicinal substance carrier.
Study Funding. This work was performed in the framework of the PhD program in Medicine (6D110100).
Acknowledgments. The authors would like to thank the administration of A.B. Bekturov Institute of Chemical Sciences and the administration of Semey State Medical University for assistance in conducting the research.
Conflicts of Interest. The authors have no conflicts of interest to disclose.
References
- Sosnovskiy S.V., Boyko E.V., Kharitonova N.N. The substantiation and development of the quantative assessment system of the severity of proliferative vitreoretinopathy. Oftal’mokhirurgiya 2009; 4: 25–29.
- Il’nitskiy V.V. Vremennoe i postoyannoe episkleral’noe plombirovanie v khirurgii otsloyki setchatki, ee profilaktika. Dis. ... dokt. med. nauk [Temporary and permanent episcleral buckling in retinal detachment surgery and its prevention. DSc Thesis]. Moscow; 1995.
- Balinskaya N.R. Kombinirovannye intravitreal’nye khirurgicheskie vmeshatel’stva pri otsloyke setchatki, oslozhnennoy vitreoretinal’noy traktsiey. Dis. ... kand. med. nauk [Combined intravitreous surgery for retinal detachment complicated by vitreoretinal traction. PhD Thesis]. Moscow; 1993.
- Kochmala O.B., Zapuskalov I.V., Krivosheina O.I., Dashko I.A. Retinal detachment surgery: state of art. Vestnik oftal’mologii 2010; 126(6): 46–49.
- Zakharov V.D. Vitreoretinal’naya khirurgiya [Vitreoretinal surgery]. Moscow: Meditsina; 2003; 180 p.
- Zhurgumbaeva G.K. Vitreosineretik «Vitrenal» v khirurgii proliferativnoy vitreoretinopatii pri otsloyke setchatki. Dis. ... kand. med. nauk [Vitreosyneretic Vitrenal in surgery of proliferative vitreoretinopathy in retinal detachment. PhD Thesis]. Almaty; 2009.
- Glinchuk Ya.I. Rol’ vitrektomii v lechenii zabolevaniy glaz travmaticheskoy, degenerativnoy i vospalitel’noy etiologii. Dis. … dokt. med. nauk [The role of vitrectomy in treatment of eye diseases of traumatic, degenerative and inflammatory etiology. DSc Thesis]. Moscow; 1987.
- Zakharov V.D., Sharipova D.N., Shatskikh A.V. Possibilities of treating proliferative vitreoretinopathy in light of modern aspects of its etiology and pathogenesis. Oftal’mokhirurgiya 2006; 2: 59–65.
- Afrashi F., Erakgun T., Akkin C., Kaskaloglu M., Mentes J. Conventional buckling surgery or primary vitrectomy with silicone oil tamponade in rhegmatogenous retinal detachment with multiple breaks. Graefes Arch Clin Exp Ophthalmol 2004; 242(4): 295–300, https://doi.org/10.1007/s00417-003-0842-2.
- Christensen U., Villumsen J. Prognosis of pseudophakic retinal detachment. J Cataract Refract Surg 2005; 31(2): 354–358.
- Goezinne F., La Heij E.C., Berendschot T.T., Kessels A.G., Liem A.T., Diederen R.M., Hendrikse F. Incidence of redetachment 6 months after scleral buckling surgery. Acta Ophthalmol 2010; 88(2): 199–206, https://doi.org/10.1111/j.1755-3768.2008.01425.x.
- Heimann H., Zou X., Jandeck C., Kellner U., Bechrakis N.E., Kreusel K.M., Helbig H., Krause L., Schüler A., Bornfeld N., Foerster M.H. Primary vitrectomy for rhegmatogenous retinal detachment: an analysis of 512 cases. Graefes Arch Clin Exp Ophthalmol 2006; 244(1): 69–78, https://doi.org/10.1007/s00417-005-0026-3.
- Salicone A., Smiddy W.E., Venkatraman A., Feuer W. Management of retinal detachment when no break is found. Ophthalmology 2006; 113(3): 398–403, https://doi.org/10.1016/j.ophtha.2005.10.002.
- Kon C.H., Asaria R.H., Occleston N.L., Khaw P.T., Aylward G.W. Risk factors for proliferative vitreoretinopathy after primary vitrectomy: a prospective study. Br J Ophthalmol 2000; 84(5): 506–511, https://doi.org/10.1136/bjo.84.5.506.
- Foster R.E., Meyers S.M. Recurrent retinal detachment more than 1 year after reattachment. Ophthalmology 2002; 109(10): 1821–1827, https://doi.org/10.1016/s0161-6420(02)01182-x.
- Miki D., Hida T., Hotta K., Shinoda K., Hirakata A. Comparison of scleral buckling and vitrectomy for retinal detachment resulting from flap tears in superior quadrants. Jpn J Ophthalmol 2001; 45(2): 187–191, https://doi.org/10.1016/s0021-5155(00)00377-4.
- Sharma Y.R., Karunanithi S., Azad R.V., Vohra R., Pal N., Singh D.V., Chandra P. Functional and anatomic outcome of scleral buckling versus primary vitrectomy in pseudophakic retinal detachment. Acta Ophthalmol Scand 2005; 83(3): 293–297, https://doi.org/10.1111/j.1600-0420.2005.00461.x.
- Yang H., Wang R., Gu Q., Zhang X. Feasibility study of chitosan as intravitreous tamponade material. Graefes Arch Clin Exp Ophthalmol 2008; 246(8): 1097–1105, https://doi.org/10.1007/s00417-008-0813-8.
- Lazarenko V.I., Bolshakov I.N., Ilyenkov S.S., Shatilova R.I., Kuzovnikov V.V., Chanchikov D.G., Osipova O.V., Gar’kavenko V.V., Simko I.V., Ivanov V.V. Using Bol-Hit and Kollahit-Bol grafts in ophthalmology. Rossiyskiy oftal’mologicheskiy zhurnal 2009; 2(4): 21–24.
- Kuzovnikov V.V., Gar’kavenko V.V., Chanchikov D.G., Osipova O.V., Ivanov V.V., Simko I.V., Lazarenko V.I., Bol’shakov I.N. Ispol’zovanie izdeliy meditsinskogo naznacheniya na osnove khitozana v oftal’mologii. V kn.: Materialy IX Mezhdunarodnoy konferentsii “Sovremennye perspektivy v issledovanii khitina i khitozana” [Use of products of medical appointment on the basis of chitosane in ophthalmology. In: Proceedings of the IX International conference “Modern perspectives in chitin and chitosan studies”]. Moscow: VNIRO Publishing; 2008; p. 181–183.
- Sharipova D.N. Profilaktika proliferativnoy vitreoretinopatii s ispol’zovaniem 5-ftoruratsila na gidrogelevom implantate. Dis. ... kand. med. nauk [Prevention of proliferative vitreoretinopathy with 5-fluorouracil on hydrogel implant. PhD Thesis]. Moscow; 2006.
- Artem’eva O.V., Samoilov A.N., Zhernakov S.V. Proliferative vitreoretinopathy: modern view on etiology and pathogenesis. Vestnik oftal’mologii 2014; 130(3): 67–71.