Morphological Reconstruction of Main Arteries by Perivascular Implantation of Sulfated Chitosan in Experimental Atherosclerosis
The aim of the study is to demonstrate morphological reconstruction of the main arteries of rabbit hind limbs by perivascular implantation of sulfated chitosan in an atherosclerosis model.
Materials and Methods. The study was performed on 24 rabbits divided into four groups of 6 animals. The rabbits in the experimental group kept on intensive cholesterol diet for 110 days were implanted 1% water-soluble sulfated chitosan gel in the perivascular fascial compartment of the saphenous artery and the femoral artery of the left hind limb. Some animals that were also kept on cholesterol diet did not undergo implantation. The group of rabbits fed with normal vivarium diet were subjected to perivascular implantation of the biopolymer, while a group of 6 rabbits also kept on normal diet served as the intact control. We estimated wall thickness of the femoral artery and the saphenous artery, their average lumen diameter, the mean lumen area of the newly formed vessels in the adventitia.
Results. Daily intensive cholesterol diet for 3.5 months in rabbits leads to vivid signs of atherogenic inflammation forming in the intimal and medial layers of the main arteries of the hind limbs. Introduction of 1% water-soluble sulfated chitosan gel into the paravasal compartment of the saphenous and femoral arteries promotes formation of a large number of microvessels at the polymer resorption site increasing the specific area of new vessels. Morphological reconstruction of the main arteries is achieved through reducing the vascular wall thickness, increasing the vessel lumen and the number of para-adventitial microvessels.
Conclusion. Implantation of sulfated chitosan into the perivascular compartment allows achieving the effect of therapeutic paravasal angiogenesis and inhibiting the early signs of atherogenic inflammation.
Atherosclerosis is considered to be one of the leading causes of morbidity and mortality in developed countries [1]. Its most common clinical and morphological form is atherosclerosis of lower limb arteries leading to disability and associated with high risk of death [1–5]. New methods of atherosclerosis prevention and treatment are developed through managing the process of atherogenic inflammation. The special role in the pathogenesis of atherosclerosis is given to the adventitia [6, 7]. This vessel wall component is considered to be as important as the medial layer and the intima because it takes part in regulating the proliferation of smooth muscle cells of the medial and inner vessel layers during atherogenesis [8–11].
Recently, there have been published a fair number of works demonstrating the positive effect of using chitosan derivatives in treatment of certain diseases, including atherosclerosis, due to high affinity of the biopolymer molecule to low and very low density lipoproteins. However, in numerous publications, the cholesterol-lowering effect of chitosan was demonstrated with enteral introduction of the biopolymer or in test studies in vitro. In addition to this effect, various chitosan-containing medications promote angiogenesis in ischemic tissues, which leads to improved blood flow [12–14]. However, the problem of managing atherogenic inflammation in the walls of main arteries remains unsolved as there is no technology of targeted transporting the biopolymers having affinity for cholesterol to the area of segmental lesions to launch simultaneously both the mechanism of therapeutic angiogenesis and the mechanism of soft plaque resorption in the sub-intimal layer of the vessel.
For more than a hundred years, the cholesterol model of atherosclerosis in experimental animals has been considered the best option providing opportunities to study the early signs of atherogenic inflammation in the vessel walls. Keeping chinchilla rabbits on cholesterol diet for 80–110 days provides significant increase in plasma triglycerides, cholesterol, LDL and VLDL lipoproteins, which is associated with high levels of lipid fractions in the walls of main arteries [15, 16].
The aim of the study is to demonstrate morphological reconstruction of the main arteries of rabbit hind limbs by perivascular implantation of sulfated chitosan in an atherosclerosis model.
Materials and Methods. The study was performed on 24 male chinchilla rabbits weighing 3.5±0.5 kg. The work was performed in accordance with ethical principles established by European Convention for the Protection of Vertebrate Animals used for Experimental and Other Scientific Purposes (the Convention was passed in Strasbourg, March 18, 1986, adopted in Strasburg, June 15, 2006). All manipulations with the animals were conducted in accordance with the regulations specified in the Guide for the Care and Use of Laboratory Animals (National Research Council, 2011). The work was approved by Ethics Committee of Krasnoyarsk State Medical University named after Prof. V.F. Voino-Yasenetsky.
Half of the animals received standard vivarium diet for 110 days. The other half received cholesterol diet in order to create an experimental model of atherogenic inflammation. The diet included the following ingredients: 0.8 g of cholesterol in unrefined vegetable oil per 1 kg of rabbit body weight. The rabbits received this mixture daily throughout the experiment, water access was free. Once a week, the diet was supplemented with raw vegetables. On day 80 of the experiment, six rabbits kept on cholesterol diet and six rabbits receiving standard vivarium diet underwent perivascular implantation of 1% water-soluble sulfated chitosan gel (β1,4D-glucopyranoside-N-methyl-sulfonate sodium, molecular weight 250 kDa, deacetylation degree 85%).
The technology for implantation of the biopolymer consisted of the following: incision of the skin and subcutaneous fat was performed in rabbits under general anesthesia in the lower third of the tibia in the projection of the main neurovascular bundle; 1% sulfated water-soluble chitosan gel was introduced into the fascial compartment of the saphenous artery in the amount of 4 ml using a 5 ml syringe with a plastic cannula with a view to the product spreading in the femoral artery bed to the inguinal fold level. The wound was closed with one-row sutures, aseptic gauze dressing applied. The dressing was changed daily with antibacterial agents to heal the skin wound.
To carry out the study, all the rabbits were divided into four groups of 6 animals. The animals of group 1 received normal vivarium diet and served as the intact control. Group 2 was kept on cholesterol diet and did not undergo implantation of the biopolymer. The animals of group 3 fed with normal vivarium diet underwent implantation of sulfated chitosan introduced into the perivascular fascial compartment of the left hind limb arteries in the amount of 4 ml. Similarly, the animals of group 4 kept on cholesterol diet were implanted chitosan.
The experimental conditions were similar for rabbits of control and experimental groups. All the animals were removed from the experiment on day 110. Soft tissue samples of the femur and tibia of both hind limbs (including the main neurovascular bundle) were harvested to perform histological examination. The tissue samples were fixed in 10% neutral buffered formalin, embedded in paraffin according to the standard technique, 3–4 µm thick histological sections being produced with subsequent hematoxylin and eosin staining.
The micro-specimens were subjected to survey microscopy and morphometric evaluation using JMicroVision v. 1.2.7 software. Quantitative evaluation included measuring the wall thickness of the femoral artery and the saphenous artery, their average lumen diameter and area. Each parameter was measured in 5 sections at 100x magnification. Photomicrographs were obtained using Axio Imager A1 microscope with AxioCam MRc5 photography system and Axio Vision software (Carl Zeiss, Germany) at magnification of 100, 200, and 400.
Statistical data processing was performed using SPSS20 software. Descriptive statistics was presented in absolute values and statistical coefficients. To assess normality of distribution, Shapiro–Wilk W test and Kolmogorov–Smirnov criteria were used. For non-parametric characteristics, descriptive statistics was presented by median and percentiles (Me [25; 75]). The overall intergroup comparison was performed according to the Kruskal–Wallis test and subsequent pairwise comparison was carried out using Mann–Whitney U test. The differences were considered statistically significant at p<0.05.
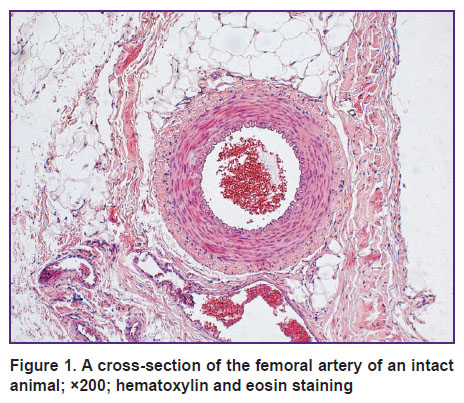
Results. Survey microscopy showed that artery walls of the intact animals had regular structure, uniform thickness (Figure 1). The adventitia was composed of loose fibrous connective tissue without clear boundaries merging into the perivascular connective tissue with numerous small vessels.
| Figure 1. A cross-section of the femoral artery of an intact animal; ×200; hematoxylin and eosin staining |
Morphological changes in the artery walls of experimental animals in group 2 revealed during experimental modeling of atherogenic inflammation evidenced the initial stages of the latter. The intima on the cross sections of the vessels had disrupted lining due to local destruction of endothelial cells (Figure 2). Undestroyed endothelial cells had unevenly located hyperchromatic nuclei protruding into the vascular lumen. The walls were segmentally thickened by plaques with the basis formed by actively proliferating fibroblasts and smooth muscle cells of the sub-endothelium. The nuclei of the latter were arranged in these sites in several rows perpendicular to the inner surface of the vessel. The fibrous plaque structure had loose appearance being the evidence of mucoid edema. The intima was devoid of wrinkles; the internal elastic membrane was displaced towards the interior of the vascular wall. The middle layer of the arteries was thinned under the plaque. Three random observations at the site of arterial wall thickening showed that individual myocytes of tunica media had vacuolated, optically empty cytoplasm, which confirmed their transformation into xanthoma cells (Figure 3). Loose fibrous connective tissue of the adventitia contained unevenly plethoric narrow blood vessels located in small groups.

|
Figure 3. Experimental atherogenic inflammation; xanthoma cells in the artery wall (arrows); ×200; hematoxylin and eosin staining |
Implantation of polysaccharide biopolymer in the perivascular tissue was accompanied by local tissue response in all cases in groups 3 and 4. By the time of removing the animals from the experiment the response had the signs of chronic productive inflammation around the foreign bodies. The implanted biopolymer underwent partial resorption in both groups. Its small deposits were scattered in the areas of various sizes. At the sites of their location, there was accumulation of macrophages in isolated small groups located occasionally along the intertissue spaces in the form of sleeves around nerve fibers and blood vessels. In the composition of macrophage infiltrates there were xanthoma cells with polygonal shape, a small hyperchromatic nucleus and weakly basophilic “foamy” appearance of the cytoplasm. Equally represented were multinucleated giant cells characterized by intensely colored amphophilic cytoplasm containing randomly distributed hyperchromatic nuclei of elongated and rounded shape (Figure 4). Lymphocytes were present in the infiltrates in smaller quantities than macrophages.

|
Figure 4. Abundance of giant multinucleated cells at the site of introducing the polysaccharide biopolymer in the perivascular layer (arrows); ×200; hematoxylin and eosin staining |
Productive macrophage tissue reaction was accompanied by appearance of numerous small blood vessels being an integral part of inflammatory foci in the animals of both groups after implantation of the biopolymer (Figure 5). Xanthoma and multinucleated cells, active macrophages bordered the entire circumference of the femoral artery in most cases (Figure 6). In one case, macrophage infiltrate was in contact with the external part of the artery wall which was noticeably thin in this area.
The external elastic membrane in the form of weakly eosinophilic homogeneously structured line containing fibrocyte-like, undifferentiated cells and newly formed small blood vessels served as the outer boundary of the arteries in both groups of rabbits after implantation. The contours of the outer vessel boundary were blurred. Smooth muscle cells in plaque composition had nuclei of elongated shape, smaller distribution density. Active development of perivascular network of vessels is the distinguishing characteristic of observations in the groups of animals subjected to implantation of the biopolymer (Figure 7).
The morphometric analysis of rabbit femoral and tibial artery slices showed that vascular wall thickness of the saphenous artery in the intact animals (group 1) was statistically significantly smaller than in rabbits kept on cholesterol diet for 110 days (group 2) and equaled 150.80 [130.38; 177.17] vs 608.33 [545.38; 748.52] µm (p<0.01). However, statistically significant reduction in vascular wall thickness to 307.49 [257.48; 334.10] µm (p<0.01) was observed in the animals kept on cholesterol diet (group 4) after implantation of 1% sulfated chitosan gel. Artery wall thickness in rabbits receiving standard vivarium diet (group 3) was 251.22 [225.72; 274.74] µm after implantation (p<0.01) (Figure 8).

|
Figure 8. Comparison of femoral arterial wall thickness (intima + media) in the animals under study |
Changes in the mean vascular wall diameter in the groups were found to be statistically significant. Thus, in the intact group, this parameter was 214.35 [201.51; 246.64] µm, while in the group receiving cholesterol diet without implantation of the biopolymer, it equaled 137.58 [124.38; 141.14] µm (p<0.05). After implantation of 1% sulfated chitosan gel in rabbits having received cholesterol diet, there was observed statistically significant increase in the mean main vessel diameter which was 163.39 [157.32; 170.83] µm (p<0.05). This parameter differed from the value in the control group less significantly (201.32 [189.26; 214.57] µm (p<0.05)) in the animals on standard vivarium diet without implantation of the biopolymer.
Implantation of 1% water-soluble sulfated chitosan gel in the paravasal space of the animals on cholesterol diet led to the increase in the lumen area of additional paravasal vessels compared to the group receiving cholesterol diet without implantation of the polymer (421.52 [409.89; 435.11] and 294.16 [280.67; 315.21] µm, respectively (p<0.05)).
At the same time, there was observed active proliferation of collateral vessels near the arterial trunk after the implantation of the biopolymer in the animals on standard vivarium diet, which led to improved perfusion of soft tissues and was considered to be a positive result of therapeutic angiogenesis.
Discussion. The obtained results offer the possibility to assume that experimental atherogenic inflammation in rabbit arteries presents morphologically in the formation of a typical lipid (“soft”) plaque covered with fibrous membrane and causing vessel lumen diameter to reduce. In the plaque mass there appear clusters of xanthoma cells, interstitial edema, subintimal and medial proliferation of smooth myocytes. On day 110 of cholesterol diet, these changes correspond to lipoidosis stage and are consistent with previously published data [4, 7, 17, 18].
Morphological changes in the artery walls caused by implantation of the polysaccharide biopolymer add up to the reduced proliferation activity of smooth muscle elements, reduced edema and, consequently, arterial wall thickness, which is objectively confirmed by morphometry results. Since the adventitia is functionally associated with the medial and intimal layers of the main vessels and actively involved in atherogenic inflammation, it is important that the adventitial and para-adventitial layers occupy the main place in therapeutic intervention and medication delivery allowing effective management of the inflammatory process, including atherogenesis [10]. In addition to achieving the effect of therapeutic angiogenesis, chitosan copolymers create direct contact with atherogenic products in the sub-endothelial space of the main arteries due to electrostatic, concentrating and affine interactions, i.e. with cholesterol-containing molecules of both lipid nuclei of soft plaques and foam cells of the intimal layer. The experiments have shown that introduction of biopolymers in the adventitial area during open or closed surgery provides the possibility to inhibit intimal thickening, inhibit restenosis, negative remodeling [9].
Thus, perivascular introduction of polysaccharide biopolymer chitosan offers opportunities to shift atherogenic inflammation center from sub-intimal to periadventitial area taking into account the great importance of increased neo-angiogenesis in the region of inflammation around the foreign body, which contributes to better oxygenation of ischemic tissues. At the same time, stimulation of angiogenesis accompanied by activation of such factors as vascular endothelial growth factor and fibroblast growth factor may induce proliferation of the endothelium and formation of vascularized atheromatous foci, i.e. progression of atherogenesis [19].
Conclusion. The technology of implantation of 1% water-soluble sulfated chitosan gel into the perivascular compartment of the main arteries of limb in experimental atherosclerosis allows achieving the effect of therapeutic paravasal angiogenesis and inhibiting the early signs of atherogenic inflammation. This permits to consider the proposed method to be promising in treatment of chronic lower limb ischemia caused by atherosclerosis. The mechanisms underlying the inhibition of atherogenesis by obvious productive peri- and paravascular inflammatory process require detailed morphological and molecular marker analysis.
Study Funding. The study was supported by Russian Foundation for Basic Research, Krasnoyarsk Region Government, Krasnoyarsk Region Foundation for Scientific and Applied Research Activity Support within the framework of scientific project No.16-44-240506.
References
- Aronov D.M. Social aspects of atherosclerosis and methods of its treatment. Russkiy meditsinskiy zhurnal 2000; 7: 276.
- Andozhskaya Yu.S., Girina M.B, Vasina E.Yu. Present-day methods of microcirculation estimate in efferent therapy of patients with atherosclerosis. Regionarnoe krovoobrashchenie i mikrotsirkulyatsiya 2002; 1(1): 52–59.
- Karpov Yu.A., Sorokin E.V. Intensive medical treatment of patients with atherosclerosis. Kardiologiya 2005; 45(8): 4–7.
- Klimenko E.D., Kobozeva L.P., Michunskaia A.B., Khabarina I.Iu. Function of the microcirculatory system during the long-term regression of the early stages of atherogenesis. Biull Eksp Biol Med 1988; 105(3): 365–368.
- Dua A., Lee J.C. Epidemiology of peripheral arterial disease and critical limb ischemia. Tech Vasc Interv Radiol 2016; 19(2): 91–95, https://doi.org/10.1053/j.tvir.2016.04.001 .
- Auger F.A., D’Orléans-Juste P., Germain L. Adventitial contribution to vascular contraction: hints provided by tissue-engineered substitutes. Cardiovasc Res 2007; 75(4): 669–678, https://doi.org/10.1016/j.cardiores.2007.06.001.
- Laflamme K., Roberge C.J., Grenier G., Rémy-Zolghadri M., Pouliot S., Baker K., Labbé R., D’Orléans-Juste P., Auger F.A., Germain L. Adventitia contribution in vascular tone: insights from adventitia-derived cells in a tissue-engineered human blood vessel. FASEB J 2006; 20(8): 1245–1247, https://doi.org/10.1096/fj.05-4702fje.
- Ni W., Kitamoto S., Ishibashi M., Usui M., Inoue S., Hiasa K., Zhao Q., Nishida K., Takeshita A., Egashira K. Monocyte chemoattractant protein-1 is an essential inflammatory mediator in angiotensin II-induced progression of established atherosclerosis in hypercholesterolemic mice. Arterioscler Thromb Vasc Biol 2004; 24(3): 534–539, https://doi.org/10.1161/01.atv.0000118275.60121.2b.
- Nugent H.M., Sjin R.T., White D., Milton L.G., Manson R.J., Lawson J.H., Edelman E.R. Adventitial endothelial implants reduce matrix metalloproteinase-2 expression and increase luminal diameter in porcine arteriovenous grafts. J Vasc Surg 2007; 46(3): 548–556, https://doi.org/10.1016/j.jvs.2007.04.074.
- Pagano P.J., Gutterman D.D. The adventitia: the outs and ins of vascular disease. Cardiovasc Res 2007; 75(4): 636–639, https://doi.org/10.1016/j.cardiores.2007.07.006.
- Rey F.E., Pagano P.J. The reactive adventitia: fibroblast oxidase in vascular function. Arterioscler Thromb Vasc Biol 2002; 22(12): 1962–1971, https://doi.org/10.1161/01.atv.0000043452.30772.18.
- Bolshakov I.N., Shestakova L.A., Kotikov A.R., Kaptyuk G.I. Experimental atherosclerosis in rats. morphological reconstruction of the main artery wall with the polyssacharide biopolymers. Fundamental’nye issledovaniya 2013; 10–3: 557–563.
- Kim S., Kawai T., Wang D., Yang Y. Engineering a dual-layer chitosan-lactide hydrogel to create endothelial cell aggregate-induced microvascular networks in vitro and increase blood perfusion in vivo. ACS Appl Mater Interfaces 2016; 8(30): 19245–19255, https://doi.org/10.1021/acsami.6b04431.
- Lee S., Valmikinathan C.M., Byun J., Kim S., Lee G., Mokarram N., Pai S.B., Um E., Bellamkonda R.V., Yoon Y.S. Enhanced therapeutic neovascularization by CD31-expressing cells and embryonic stem cell-derived endothelial cells engineered with chitosan hydrogel containing VEGF-releasing microtubes. Biomaterials 2015; 63: 158–167, https://doi.org/10.1016/j.biomaterials.2015.06.009.
- Bolshakov I.N., Dolgikh O.A., Kirichenko A.K., Kotikov A.R., Gorbunova V.O. Lipid spectrum and microcirculation when using biopolymers in an atherogenesis model. Fundamental’nye issledovaniya 2009; S7: 41–42.
- Klimov A.N., Parfenova N.S., Golikov Y.P. One century of the cholesterol model of atherosclerosis. Biomeditsinskaya khimiya 2012; 58(1): 5–11.
- Bolshakov I.N., Shestakova L.A., Kotikov A.R., Kaptyuk G.I. The experimental atherosclerotic inflammation of the main arteries in rabbits. Low traumatic technology of morphological reconstruction of the vascular wall at the early atherosclerotic stages. Fundamental’nye issledovaniya 2013; 8–2: 343–350.
- Dzyak G.V., Koval’ E.L. Atherosclerosis and inflammation. Problema stareniya i dolgoletiya 1999; 3: 316–326.
- Liu M.H., Tang Z.H., Li G.H., Qu S.L., Zhang Y., Ren Z., Liu L.S., Jiang Z.S. Janus-like role of fibroblast growth factor 2 in arteriosclerotic coronary artery disease: аtherogenesis and angiogenesis. Atherosclerosis 2013; 229(1): 10–17, https://doi.org/10.1016/j.atherosclerosis.2013.03.013.