Composite Tissue-Engineered Small-Diameter Vascular Grafts Based on Polycaprolactone and Polyurethane with Growth Factors and Atrombogenic Drug Coatings: Surface Ultrastructure, Physical and Mechanical Properties
There is a growing need for synthetic small-diameter vascular grafts (<6 mm) for bypass surgery since the majority of currently developed products have demonstrated unacceptable high frequency of thrombosis in preclinical studies. The proprietary composite vascular graft based on a nonwoven polymer with anti-thrombogenic and anti-aneurysm effect and functional activity is aimed at stimulating the formation of vascular neotissue at the implantation site.
The aim of the investigation is to study the surface morphology, physical and mechanical properties of the polycaprolactone/polyurethane (PCL/PU) small-diameter tissue-engineered vascular graft with growth factors and an anti-thrombogenic surface coating.
Materials and Methods. PCL/PU vascular grafts with growth factor mix (GFmix) were manufactured using the electrospinning method. The hydrogel coating containing iloprost (Ilo) and heparin (Hep) was formed by complexation with polyvinylpyrrolidone. The controls were multilayer vascular grafts of similar composition and nonwoven matrices based on 12% PCL and 12% PU. The surface structure was analyzed with the S-3400N scanning electron microscope (Hitachi, Japan). The physical properties of the surface were determined by the wetting angle method. The mechanical properties were evaluated on a Z series universal testing machine (Zwick/Roell, Germany). Statistical processing of the data was performed using the GraphPad Prism 8 software.
Results. Our new manufacturing technique for the composite PU/PCL/GFmix/Ilo/Hep graft has eliminated the problem of graft delamination. The inner surface of the graft consisted of interwined microfibers (1.34 [1.15; 2.28] μm thick), nanofibers (790.0 [604.0; 853.5] nm thick), and interpenetrating pores of different diameters (5.4 [3.8; 8.4] μm). The process of coating formation did not affect the fibers and did not seal the pores, the surface retained its hydrophilic properties (θ=68.61±11.85°). The tensile strength (3.45 [3.17; 4.03] МPа) and Young’s modulus (4.88 [3.95; 5.80] MPa) of PU/PCL/GFmix/Ilo/Hep grafts were almost similar to the human internal thoracic artery compared to the multilayer analogs. The PU/PCL/GFmix/Ilo/Hep grafts were superior to the multilayer PCL/PU/GFmix/Ilo/Hep grafts in terms of reduced excessive elasticity (to 118.0 [111.0; 125.0]%; р=0.043).
Conclusion. The composite functionalized vascular PU/PCL/GFmix/Ilo/Hep grafts have enhanced characteristics and compliance, which, in turn, increases the probability of their high patency in future preclinical studies.
Introduction
Vascular diseases are one of the leading causes of disability and mortality in the world. The standard treatment for severe vascular diseases is bypass surgery using autologous arteries or veins, however, their application is limited due to a number of factors [1–3]. The main synthetic materials for vascular reconstruction of blood vessels such as Gortex, Dacrone, and ePTFE (d>6 m) are prone to neointimal hyperplasia, calcification and bacterial infection resulting in the reduction of patency of these grafts and a high risk of complete incompetence in the long-term period [4–6]. These materials are entirely unsuitable as substitutes for small-diameter vessels (d<6 mm) because of their rapid thrombosis [7–9]. Some natural commercial grafts such as Artegraft®, ProCol®, and Omniflow II® represent biosynthetic prostheses based on bovine vessels and are used as a shunt for dialysis and replacement of large-diameter vessels. These alternatives also have key limitations preventing their application in the bypass surgery on the small-caliber vessels: inconsistency of mechanical properties, the risk of aneurysm formation and/or thrombosis, allergic reactions, and rejections [10, 11].
Presently, no effective synthetic small-diameter vascular prostheses have been created for clinical applications. A tissue-engineered vascular graft fabricated using new methods of material sciences, engineering and cell biology seems to be a promising candidate. Its advantages over autografts include non-invasive surgical intervention during graft preparation, unlimited availability, and the possibility to use an individualized approach to the graft size. However, the existing developments are far from being satisfactory. Since there are a variety of unsolved questions, searching for the materials for small-diameter vascular grafts is going on [12–15]. The majority of candidates for these prostheses are manufactured by the electrospinning technique or 3D printing from a polymer solution, which makes it possible to create a fibrous porous non-woven material similar to the natural extracellular matrix. A low blood flow rate in small-caliber vessels is responsible for a high risk of thrombosis, which the researchers face at the stage of testing their items on a large animal model [16]. To overcome this and some other problems, tissue-engineered grafts may be made functionally active: for example, by stimulating endothelialization; attracting the cells synthetizing extracellular matrix components; increasing their anti-microbial and anti-thrombotic potential; it is also possible to enhance the scaffold to prevent aneurysm formation [17–19].
Our new technology for creation a functionalized vascular prosthesis has some specific features. The first is a composite base from polycaprolactone (PCL) and polyurethane (PU). PCL possesses a sufficiently high strength and elasticity and is subject to biodegradation during 2–3 years. At the same time, gradual bioresorption of PCL will provide the possibility of full graft remodeling by replacing the polymer tubular scaffold with the patient’s own cells and tissues [20]. The presence of PCL in the scaffold composition promotes the adaptive growth of the graft upon its implantation into the vascular bed. PU is a synthetic polymer with a high biocompatibility and excellent mechanical properties, which possesses increased stability in the biological systems and is used in the biomedical industry. Incorporation of this polymer into the composition of the polymer graft scaffold will bring the physical and mechanical properties of the graft closer to the properties of the native small-diameter arterial vessels and provide the resistance of its walls to aneurysmal dilatation due to extremely low rate of hydrolytic degradation [21–23]. The combination of these polymers will impart high strength and durability, elasticity and bending resistance to the vascular graft. After the implantation of this graft into the vascular bed, formation of graft wall aneurysms in the process of its functioning in the vascular bed will be prevented preserving the possibility of full remodeling.
The second feature consists in the stimulation of the graft remodeling process after the implantation into the vascular bed with angiogenic factors: vascular endothelial growth factor (VEGF), basic fibroblast growth factor (bFGF), and chemoattractant molecules — stromal cell-derived factor 1 alfa (SDF-1α). VEGF triggers endothelialization by activating migration, proliferation, and differentiation of endothelial cells. bFGF stimulates migration, proliferation, and survival of endothelial and smooth muscle cells. SDF-1α facilitates the attraction of bone marrow-derived progenitor cells from the bloodstream to the zone of the graft location [24].
The third feature involves superficial modification of the grafts with athrombogenic drugs (iloprost (Ilo) and heparin (Hep)) to prevent lumen thrombosis of the implanted vascular prostheses in the early postoperative period [25].
The quality of the material should be controlled at each stage of tissue-engineered vascular graft fabrication, since the additional modifying procedures may change its initial characteristics. This article presents data of the in vitro comparison of the new technology of fabricating a monolayer functionalized composite vascular graft with the previous multilayer graft design based on the same composition.
The aim of the study is to evaluate the surface structure, physical and mechanical characteristics of the composite tissue-engineered vascular graft of a small diameter based on polycaprolactone and polyurethane with growth factors and antithrombogenic coating, and to compare it with the multilayer analog.
Materials and Methods
Fabrication of PCL and PU scaffolds. Scaffolds were fabricated by the electrospinning technique (Nanon-01A; MECC, Japan) from the solution of 12% PCL (Sigma-Aldrich, USA) and 12% PU (Tecoflex EG-80A; Lubrizol Advanced Materials, USA) in chloroform using the following parameters: needle — 22 G, voltage — 20 kV, solution feed rate — 0.5 ml/h, collector rotation speed — 200 rpm, needle cleaning time — 30 s.
Fabrication of multilayer vascular PCL/GFmix/PU/Ilo/Hep (d=4 mm) grafts. At the first stage, the grafts were manufactured in layers using the electrospinning technique from the polymer solution in chloroform (Vekton, Russia). The first (internal) layer consisted of 12% PCL + 1% Plu (Sigma-Aldrich, USA) with incorporation of VEGF (SaiStorLab, Russia), the second layer — 12% PCL + 1% Plu (Sigma-Aldrich, USA) with incorporation of bFGF (SaiStorLab, Russia) and SDF-1α (Cloud-Сlone Сorp., USA). The parameters used were as follows: needle — 22 G, voltage — 22 kV, collector rotation speed — 200 rpm, solution feed rate — 0.5 ml/h, needle cleaning time — 30 s, distance from the needle to the winding collector — 15 cm. The third layer contained 12% PU in chloroform. The parameters were the same except the voltage, which was 20 kV.
At the second stage, a drug coating with Ilo and Hep was formed by complexation with polyvinylpyrrolidone [26].
Fabrication of composite monolayer vascular PCL/PU/GFmix/Ilo/Hep (d=4 mm) grafts. At the first stage, the grafts were fabricated using the electrospinning technique from the polymer solution in chloroform — 8% PCL + 5% PU + 1% Plu with a concomitant introduction of the growth factor complex (GFmix: VEGF, bFGF, and SDF-1α). The following parameters of electrospinning were used: voltage — 22 kV, collector rotation speed — 200 rpm, solution feed rate — 0.5 ml/h, needle cleaning time — 30 s, distance from needle to winding collector — 15 cm.
The second stage is completely identical to the one described above.
Surface ultrastructure. The structure of the layer surface was assessed using the S-3400N SEM (Hitachi, Japan) under a high vacuum at the 10 kV accelerating voltage. Prior to the test, a 15 nm gold-palladium coating was deposited (EM ACE200; Leica Mikrosysteme GmbH, Austria) on the 0.5×0.5 cm graft samples.
Physical and mechanical properties. To evaluate hydrophilic/hydrophobic properties, a wetting angle on the polymer materials was determined using the sitting drop method and the Drop Shape Analyzer DSA25 (KRÜSS GmbH, Germany) at room temperature. The contact wetting angle was calculated from 5 images in the ImageJ software (NIH, USA) for each type of the polymer material.
The mechanical properties of the material were tested on the universal series Z testing machine (Zwick/Roell, Germany) using the probe with a nominal force of 50 H and an acceptable error threshold ±1%, crossbeam moving speed during testing was 50 mm/min. The material tensile strength was determined as ultimate stress in tension (MPa) prior to destruction. Since the tested biological samples and grafts differed essentially in thickness and, consequently, a cross-section area, absolute value of the maximal force applied to the sample before the beginning of destruction (Fmax, Н) was used as an alternative criterion of strength. Elastic deformation of the material was assessed by relative elongation prior to sample destruction (%) and the Young’s modulus (MPa), which was determined in the range of physiological pressure (80–120 mm Hg). The mechanical properties of the grafts were compared with those of the human internal thoracic artery.
Statistical data processing. The results were statistically processed using GraphPad Prism 8 program (GraphPad Software, USA). The character of data distribution in the samples was evaluated by Kolmogorov–Smirnov and Shapiro–Wilk tests. The qualitative data were presented as mean ± σ or median and quartile range (Me [25%; 75%]). Statistically significant differences between independent groups were assessed using the Kruskal–Wallis test with the result correction considering multiple comparisons by the FDR method. The differences were considered significant at p<0.05 for all tests.
Results
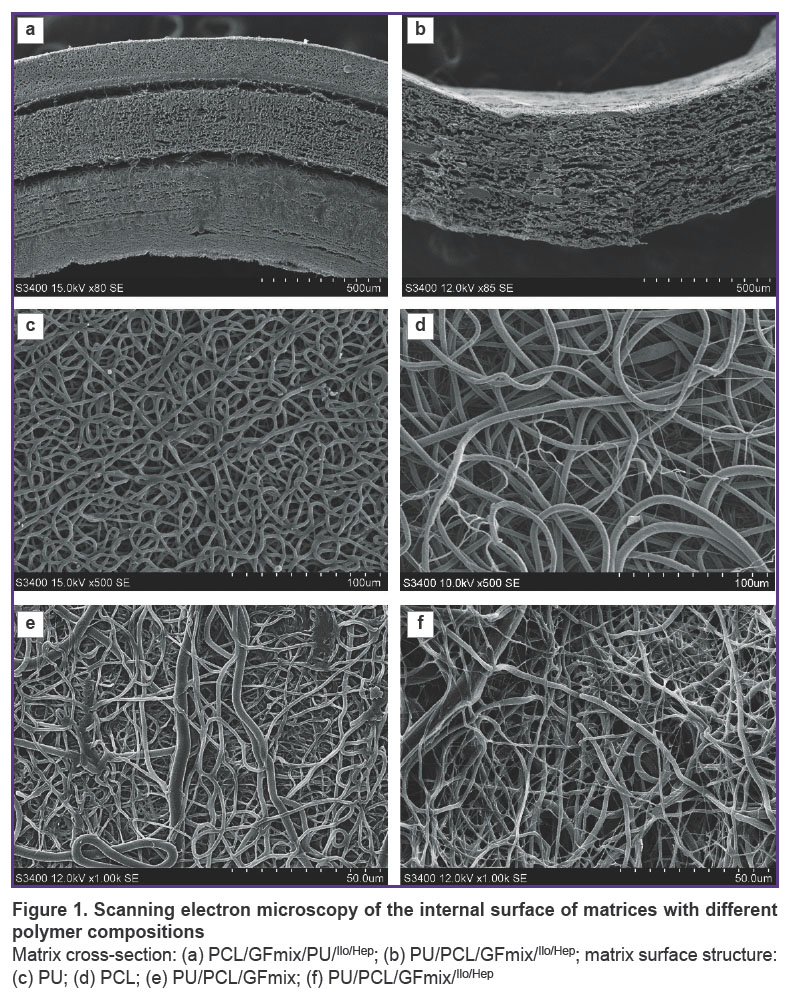
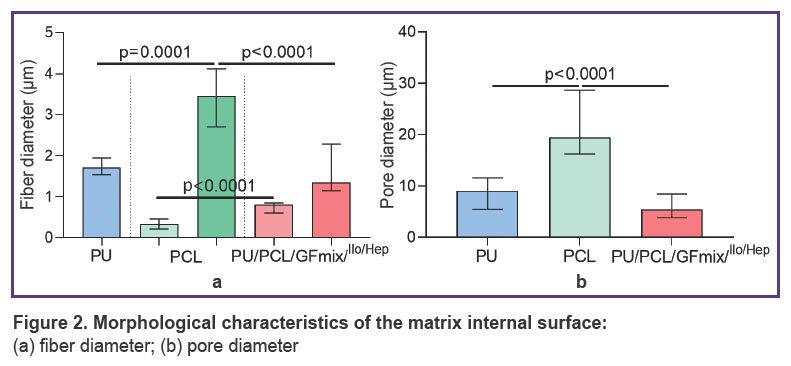
The structure of the material surface. The initial variant of fabricating a multilayer vascular graft with external PU reinforcement by the electrospinning method was characterized by the separation of the external PU layer and entire radial delamination of the PU/GFmix base due to the stepwise formation of 1/3 of the item with VEGF incorporation and 2/3 with bFGF + SDF-1α (Figure 1 (а)). The idea of combining PU, PCL and three differentiation factors in a single solution enabled us to create a tissue-engineered prosthesis without delamination of its wall (Figure 1 (b)). The PU/PCL/GFmix scaffold possessed the characteristics inherent to each monocomponent material: the internal surface consisted of interwined microfibers (1.34 [1.15; 2.28] μm thick) and nanofibers (790.0 [604.0; 853.5] nm thick) of convoluted disordered orientation with pores of different diameters (5.4 [3.8; 8.4] μm) partly compacted by the networks of tightly interwoven nanofibers. The addition of PU to PCL resulted in a statistically significant 3.6-fold reduction of the pore diameter (р<0.0001) relative to the PCL surface (19.5 [16.2; 28.6] μm). Porosity of PCL/PU/GFmix wall was not less than 50% (Figure 1 (c)–(e); Figure 2). The PVP cross-linking with the PCL ester groups under the conditions of the argon atmosphere and gamma-radiation and further processing by the drug mix with drying did not affect the fiber structure and did not seal the pores (Figure 1 (f)).
|
Figure 2. Morphological characteristics of the matrix internal surface:
(а) fiber diameter; (b) pore diameter |
Physical characteristics of the material surface. Determination of the contact wetting angle has revealed an essential increase in the hydrophilic properties of the composite PU/PCL/GFmix material (θ=31.01±13.17°) in comparison with monocomponent analogs (p<0.0001). Formation of the drug coating promoted statistically significant 2.2-fold reduction of hydrophilic properties (p<0.0001); an average angle of contact with water was equal to 68.61±11.85° (Figure 3), however, this modified surface may be considered sufficiently hydrophilic.
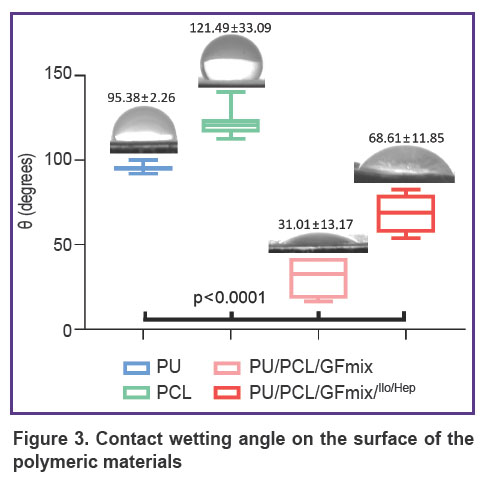
|
Figure 3. Contact wetting angle on the surface of the polymeric materials |
Mechanical characteristics of the composite vascular grafts. The drug coating on the PU/PCL/GFmix grafts is known to cause no significant effect on the mechanical characteristics of the item. We managed to bring the parameters of strain and stiffness of the PU/PCL/GFmix/Ilo/Hep closer to those of the human internal thoracic artery as compared to the PCL/GFmix/PU/Ilo/Hep grafts, in which polyurethane acted as an external reinforcement layer. It should be also noted that the composite PU/PCL/GFmix/Ilo/Hep grafts had statistically significantly lower excessive elasticity (up to 118.0 [111.0; 125.0]%) relative to the value for the PCL/GFmix/PU/Ilo/Hep grafts (p=0.043) (see the Table).
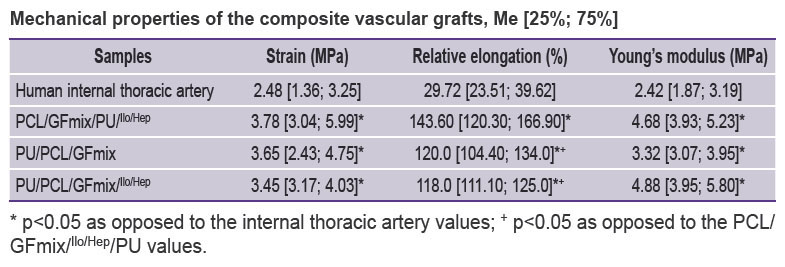
|
Mechanical properties of the composite vascular grafts, Me [25%; 75%] |
Discussion
To create tissue-engineered small-diameter vascular prostheses, numerous factors should be taken into consideration in order to achieve their effective functioning in the patient’s vascular bed. The graft must possess biomechanical characteristics as close to the native artery as possible for the appropriate compliance in the hemodynamic conditions and prevention of neointimal hyperplasia. This effect may be achieved by regulation of the electrospinning parameters or other methods used to fabricate non-woven materials. The current trend of developing vascular grafts is to enhance their functional activity, which is directed to solving the main problems holistically connected with effective patency in the long-term period and minimal need for graft replacement. In case of a low blood flow and increased resistance [27], there is a high risk of early graft thrombosis in this implantation projection, therefore, researchers modify them by various methods: reducing pores in the internal surface and creating antithrombotic layers, which may cause spontaneous endothelialization, or releasing locally drugs blocking thrombocyte attachment and thrombus formation. Concurrently with the problem of patency, the task of harmonization of the artificial scaffold remodeling also needs solving. This can be achieved by attracting a number of cells such as endothelial cells and fibroblasts to the surface and thickness of the graft wall. Implantation of tissue-engineered grafts into the vascular bed of the small and large laboratory animals makes it possible to evaluate de novo generated tissue consisting as a rule of the structures similar to the native vascular tissue: neointima, neomedia, neoadventitia, and fragments of the polymer scaffold [28]. This tissue is susceptible to aneurysm formation, which is what we observed in our experiments on the sheep model [29]. Reinforcement of the vascular graft scaffold making it resistant to bioresorption is also an important task in creating medical items of this class.
The proprietary design of the functionalized unwoven small-diameter vascular prosthesis based on polycaprolactone and polyurethane with the incorporated growth factor mix (VEGF, bFGF, SDF-1α) allowed us to obtain a graft with a highly porous structure without delamination of its wall. Formation of a hydrophilic hydrogel coating (θ=68.61±11.85°) using radiation polymerization of PVP with further complexation with Ilo and Hep did not affect the mechanical properties of the final product. The mechanical characteristics of the composite PU/PCL/GFmix/Ilo/Hep grafts were closer to those of the native artery than the parameters of the multilayer PCL/GFmix/PU/Ilo/Hep grafts and our previous designs [30] owing to the improved manufacturing technology: replacement of the polymeric component, namely, polyhydroxybutyrate/valerate with polyurethane; usage of the mix in a single polymer solution with the addition of emulsion stabilizer Pluronic instead of layered incorporation of the growth factors. This technology enabled us to create a new promising small-diameter vascular graft.
Conclusion
The composite functionalized vascular PU/PCL/GFmix/Ilo/Hep graft possesses improved characteristics and compliance, which, in turn, may increase the probability of high patency when implanted to the large laboratory animals in preclinical trials.
Research funding. The study was carried out with the financial support of the Ministry of Science and Higher Education of the Russian Federation within the framework of the Agreement on the Provision of grants from the Federal Budget in the form of subsidies in accordance with paragraph 4 of Article 78.1 of the Budget Code of the Russian Federation No.075-15-2022-1202 dated September 30, 2022, concluded in order to implement the Decree of the Government of the Russian Federation dated May 11, 2022, No.1144-r.
Conflicts of interest. The authors declare the absence of obvious and potential conflicts of interest related to the publication of this article.
References
- Tinica G., Chistol R.O., Enache M., Leon Constantin M.M., Ciocoiu M., Furnica C. Long-term graft patency after coronary artery bypass grafting: effects of morphological and pathophysiological factors. Anatol J Cardiol 2018; 20(5): 275–282, https://doi.org/10.14744/AnatolJCardiol.2018.51447.
- Virani S.S., Alonso A., Benjamin E.J., Bittencourt M.S., Callaway C.W., Carson A.P., Chamberlain A.M., Chang A.R., Cheng S., Delling F.N., Djousse L., Elkind M.S.V., Ferguson J.F., Fornage M., Khan S.S., Kissela B.M., Knutson K.L., Kwan T.W., Lackland D.T., Lewis T.T., Lichtman J.H., Longenecker C.T., Loop M.S., Lutsey P.L., Martin S.S., Matsushita K., Moran A.E., Mussolino M.E., Perak A.M., Rosamond W.D., Roth G.A., Sampson U.K.A., Satou G.M., Schroeder E.B., Shah S.H., Shay C.M.,
- Spartano N.L., Stokes A., Tirschwell D.L., VanWagner L.B., Tsao C.W.; American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics — 2020 Update: a report from the American Heart Association. Circulation 2020; 141(9): e139–e596, https://doi.org/10.1161/CIR.0000000000000757.
- Pashneh-Tala S., MacNeil S., Claeyssens F. The tissue-engineered vascular graft-past, present, and future. Tissue Eng Part B Rev 2016; 22(1): 68–100, https://doi.org/10.1089/ten.teb.2015.0100.
- Lejay A., Bratu B., Kuntz S., Neumann N., Heim F., Chakfé N. Calcification of synthetic vascular grafts: a systematic review. EJVES Vasc Forum 2023; 60: 1–7, https://doi.org/10.1016/j.ejvsvf.2023.05.013.
- Yu L., Newton E.R., Gillis D.C., Sun K., Cooley B.C., Keith A.N., Sheiko S.S., Tsihlis N.D., Kibbe M.R. Coating small-diameter ePTFE vascular grafts with tunable poly(diol-co-citrate-co-ascorbate) elastomers to reduce neointimal hyperplasia. Biomater Sci 2021; 9(15): 5160–5174, https://doi.org/10.1039/d1bm00101a.
- Zhang B., Xu Y., Ma S., Wang L., Liu C., Xu W., Shi J., Qiao W., Yang H. Small-diameter polyurethane vascular graft with high strength and excellent compliance. J Mech Behav Biomed Mater 2021; 121: 104614, https://doi.org/10.1016/j.jmbbm.2021.104614. Moreno M.J., Ajji A., Mohebbi-Kalhori D., Rukhlova M., Hadjizadeh A., Bureau M.N. Development of a compliant and cytocompatible micro-fibrous polyethylene terephthalate vascular scaffold. J Biomed Mater Res B Appl Biomater 2011; 97(2): 201–214, https://doi.org/10.1002/jbm.b.31774.
- Krivkina E.O., Antonova L.V. Results of long-term patency of small-diameter biodegradable vascular prostheses with atrombogenic drug coating of sheep model. Complex Issues of Cardiovascular Diseases 2021; 10(2): 36–39, https://doi.org/10.17802/2306-1278-2021-10-2S-36-39.
- Lee K.S., Kayumov M., Emechebe G.A., Kim D.W., Cho H.J., Jeong Y.J., Lee D.W., Park J.K., Park C.H., Kim C.S., Obiweluozor F.O., Jeong I.S. A comparative study of an anti-thrombotic small-diameter vascular graft with commercially available e-PTFE graft in a porcine carotid model. Tissue Eng Regen Med 2022; 19(3): 537–551, https://doi.org/10.1007/s13770-021-00422-4.
- Lin C.H., Hsia K., Ma H., Lee H., Lu J.H. In vivo performance of decellularized vascular grafts: a review article. Int J Mol Sci 2018; 19(7): 2101, https://doi.org/10.3390/ijms19072101.
- van de Laar B.C., van Heusden H.C., Pasker-de Jong P.C., van Weel V. Omniflow II biosynthetic grafts versus expanded polytetrafluoroethylene grafts for infrainguinal bypass surgery. A single-center retrospective analysis. Vascular 2022; 30(4): 749–758, https://doi.org/10.1177/17085381211029815.
- Antonova L.V., Sevostyanova V.V., Mironov A.V., Krivkina E.O., Velikanova E.A., Matveeva V.G., Glushkova T.V., Elgudin Ya.L., Barbarash L.S. In situ vascular tissue remodeling using biodegradable tubular scaffolds with incorporated growth factors and chemoattractant molecules. Complex Issues of Cardiovascular Diseases 2018; 7(2): 25–36, https://doi.org/10.17802/2306-1278-2018-7-2-25-36.
- Tan W., Boodagh P., Selvakumar P.P., Keyser S. Strategies to counteract adverse remodeling of vascular graft: a 3D view of current graft innovations. Front Bioeng Biotechnol 2023; 10: 1097334, https://doi.org/10.3389/fbioe.2022.1097334.
- Xie X., Wu Q., Liu Y., Chen C., Chen Z., Xie C., Song M., Jiang Z., Qi X., Liu S., Tang Z., Wu Z. Vascular endothelial growth factor attenuates neointimal hyperplasia of decellularized small-diameter vascular grafts by modulating the local inflammatory response. Front Bioeng Biotechnol 2022; 10: 1066266, https://doi.org/10.3389/fbioe.2022.1066266.
- Zhang Q., Bosch-Rué È., Pérez R.A., Truskey G.A. Biofabrication of tissue engineering vascular systems. APL Bioeng 2021; 5(2): 021507, https://doi.org/10.1063/5.0039628.
- Antonova L.V., Sevostianova V.V., Silnikov V.N., Krivkina E.O., Velikanova E.A., Mironov A.V., Shabaev A.R., Senokosova E.A., Khanova M.Y., Glushkova T.V., Akentieva T.N., Sinitskaya A.V., Markova V.E., Shishkova D.K., Lobov A.A., Repkin E.A., Stepanov A.D., Kutikhin A.G., Barbarash L.S. Comparison of the patency and regenerative potential of biodegradable vascular prostheses of different polymer compositions in an ovine model. Int J Mol Sci 2023; 24(10): 8540, https://doi.org/10.3390/ijms24108540.
- Antonova L.V., Silnikov V.N., Sevostyanova V.V., Yuzhalin A.E., Koroleva L.S., Velikanova E.A., Mironov A.V., Godovikova T.S., Kutikhin A.G., Glushkova T.V., Serpokrylova I.Y., Senokosova E.A., Matveeva V.G., Khanova M.Y., Akentyeva T.N., Krivkina E.O., Kudryavtseva Y.A., Barbarash L.S. Biocompatibility of small-diameter vascular grafts in different modes of RGD modification. Polymers (Basel) 2019; 11(1): 174, https://doi.org/10.3390/polym11010174.
- Ding K., Yu X., Wang D., Wang X., Li Q. Small diameter expanded polytetrafluoroethylene vascular graft with differentiated inner and outer biomacromolecules for collaborative endothelialization, anti-thrombogenicity and anti-inflammation. Colloids Surf B Biointerfaces 2023; 229: 113449, https://doi.org/10.1016/j.colsurfb.2023.113449.
- Matsushita H., Hayashi H., Nurminsky K., Dunn T., He Y., Pitaktong I., Koda Y., Xu S., Nguyen V., Inoue T., Rodgers D., Nelson K., Johnson J., Hibino N. Novel reinforcement of corrugated nanofiber tissue-engineered vascular graft to prevent aneurysm formation for arteriovenous shunts in an ovine model. JVS Vasc Sci 2022; 3: 182–191, https://doi.org/10.1016/j.jvssci.2022.01.002.
- Siddiqui N., Asawa S., Birru B., Baadhe R., Rao S. PCL-based composite scaffold matrices for tissue engineering applications. Mol Biotechnol 2018; 60(7): 506–532, https://doi.org/10.1007/s12033-018-0084-5.
- Kucinska-Lipka J., Gubanska I., Janik H., Sienkiewicz M. Fabrication of polyurethane and polyurethane based composite fibres by the electrospinning technique for soft tissue engineering of cardiovascular system. Mater Sci Eng C Mater Biol Appl 2015; 46: 166–176, https://doi.org/10.1016/j.msec.2014.10.027.
- Tatai L., Moore T.G., Adhikari R., Malherbe F., Jayasekara R., Griffiths I., Gunatillake P.A. Thermoplastic biodegradable polyurethanes: the effect of chain extender structure on properties and in-vitro degradation. Biomaterials 2007; 28(36): 5407–5417, https://doi.org/10.1016/j.biomaterials.2007.08.035.
- Hergenrother R.W., Wabers H.D., Cooper S.L. Effect of hand segment chemistry and strain on the stability of polyurethanes: in vivo biostability. Biomaterials 1993; 14(6): 449–458, https://doi.org/10.1016/0142-9612(93)90148-u.
- Antonova L.V., Sevostyanova V.V., Mironov A.V., Krivkina E.O., Velikanova E.A., Matveeva V.G., Glushkova T.V., Elgudin Ya.L., Barbarash L.S. In situ vascular tissue remodeling using biodegradable tubular scaffolds with incorporated growth factors and chemoattractant molecules. Complex Issues of Cardiovascular Diseases 2018; 7(2): 25–36, https://doi.org/10.17802/2306-1278-2018-7-2-25-36.
- Matsuzaki Y., Miyamoto S., Miyachi H., Iwaki R., Shoji T., Blum K., Chang Y.C., Kelly J., Reinhardt J.W., Nakayama H., Breuer C.K., Shinoka T. Improvement of a novel small-diameter tissue-engineered arterial graft with heparin conjugation. Ann Thorac Surg 2021; 111(4): 1234–1241, https://doi.org/10.1016/j.athoracsur.2020.06.112.
- Antonova L.V., Sevostyanova V.V., Rezvova M.A., Krivkina E.O., Kudryavtseva Y.A., Barbarash O.L., Barbarash L.S. Technology of producing functionally active biodegradable small-diameter vascular prostheses with drug coating. Patent RU 2702239C1. 2019.
- Rickel A.P., Deng X., Engebretson D., Hong Z. Electrospun nanofiber scaffold for vascular tissue engineering. Mater Sci Eng C Mater Biol Appl 2021; 129: 112373, https://doi.org/10.1016/j.msec.2021.112373.
- Zhao L., Li X., Yang L., Sun L., Mu S., Zong H., Li Q., Wang F., Song S., Yang C., Zhao C., Chen H., Zhang R., Wang S., Dong Y., Zhang Q. Evaluation of remodeling and regeneration of electrospun PCL/fibrin vascular grafts in vivo. Mater Sci Eng C Mater Biol Appl 2021; 118: 111441, https://doi.org/10.1016/j.msec.2020.111441.
- Antonova L.V., Krivkina E.O., Sevostianova V.V., Mironov A.V., Rezvova M.A., Shabaev A.R., Tkachenko V.O., Krutitskiy S.S., Khanova M.Y., Sergeeva T.Y., Matveeva V.G., Glushkova T.V., Kutikhin A.G., Mukhamadiyarov R.A., Deeva N.S., Akentieva T.N., Sinitsky M.Y., Velikanova E.A., Barbarash L.S. Tissue-engineered carotid artery interposition grafts demonstrate high primary patency and promote vascular tissue regeneration in the ovine model. Polymers (Basel) 2021; 13(16): 2637, https://doi.org/10.3390/polym13162637.
- Antonova L.V., Krivkina E.O., Silnikov V.N., Gruzdeva O.V., Rezvova M.A., Akentieva T.N., Glushkova T.V., Tkachenko V.O., Sakharova V.M., Barbarash L.S. Evaluation of the biocompatibility and antimicrobial properties of biodegradable vascular grafts of various polymer composition with atrombogenic and antimicrobial drug coating. Vestnik transplantologii i iskusstvennyh organov 2021; 23(2): 122–136, https://doi.org/10.15825/1995-1191-2021-2-122-136.