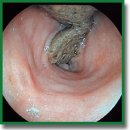
The Model of Crohn’s Disease on Large Laboratory Animals — Pigs
Dissatisfactory results of treating inflammatory bowel disease require the development of new modern methods of therapy.
The aim of the study is to create a model of Crohn’s disease on large animals, in which ulcerative defects can be formed, for testing new treatment techniques and assessing their effectiveness by endoscopic and morphological methods.
Materials and Methods. The model was created and tested on 12 castrated male pigs (hybrids between the Wiesenau and the Vietnamese black potbellied pigs), aged 6 months. The animals were manipulated under general sedation in the operating room of the SPF-vivarium for large laboratory animals at Privolzhsky Research Medical University (Russia). Endoscopic techniques and a high-frequency electrosurgical apparatus were used to create the required defects. The results were assessed endoscopically and with histological and morphometrical techniques on days 7, 14, and 21.
Results. The morphological examination of the pigs’ intestinal mucous membrane has detected the signs typical of Crohn’s disease, demonstrating the possibility of using pigs as a model of ulcerative defects in Crohn’s disease.
Conclusion. This model of Crohn’s disease on large animals (pigs, in particular) significantly widens the borders of using new treatment techniques at the preclinical stage and will improve therapy effectiveness in patients with this disease reducing the risk of surgical intervention.
- Bharadwaj S., Narula N., Tandon P., Yaghoobi M. Role of endoscopy in inflammatory bowel disease. Gastroenterol Rep (Oxf) 2018; 6(2): 75–82, https://doi.org/10.1093/gastro/goy006.
- Domènech E., Mañosa M., Cabré E. An overview of the natural history of inflammatory bowel diseases. Dig Dis 2014; 32(4): 320–327, https://doi.org/10.1159/000358131.
- Wang J.Q., Huang Y. Serological markers of inflammatory bowel disease. World Chinese J Dig 2013; 21(36): 4110, https://doi.org/10.11569/wcjd.v21.i36.4110.
- Gajendran M., Loganathan P., Catinella A.P., Hashash J.G. A comprehensive review and update on Crohn’s disease. Dis Mon 2018; 64(2): 20–57, https://doi.org/10.1016/j.disamonth.2017.07.001.
- Iborra M., Maroto N., Navarro-Cortes P., Beltran B., Boscá-Watts M., Ferrer I., Garcia-Morales N., Sáez-González E., Hinojosa J., Minguez M., Nos P. P714 vedolizumab, an adequate option in medically refractory and thiopurine-intolerant inflammatory bowel disease patients. Journal of Crohn’s and Colitis 2018; 12: S472–S473, https://doi.org/10.1093/ecco-jcc/jjx180.841.
- Gutiérrez A., Sempere L., Belvis M., Vázquez J.M., Laveda R., García M., Argüelles-Arias F., Pallarés H., Castro L., Gómez E., Maldonado B. P381 predictors of vedolizumab response to induction: real-life experience. Journal of Crohn’s and Colitis 2018; 12: S297–S297, https://doi.org/10.1093/ecco-jcc/jjx180.508.
- Iborra M., Beltrán B., Maroto N., Navarro-Cortés P., Boscá-Watts M., Ferrer-Bradley I., García-Morales N., Sáez-González E., Hinojosa J., Mínguez M., Nos P. Vedolizumab, an option in patients with inflammatory bowel disease intolerant to thiopurines and refractory to biological agents. Gastroenterol Hepatol 2018; 41(9): 535–543, https://doi.org/10.1016/j.gastrohep.2018.06.001.
- Li Y., Altemus J., Lightner A.L. Mesenchymal stem cells and acellular products attenuate murine induced colitis. Stem Cell Res Ther 2020; 11(1): 515, https://doi.org/10.1186/s13287-020-02025-7.
- Baik S.H., Kim W.H. A comprehensive review of inflammatory bowel disease focusing on surgical management. J Korean Soc Coloproctol 2012; 28(3): 121–131, https://doi.org/10.3393/jksc.2012.28.3.121.
- Alemany-Cosme E., Sáez-González E., Moret I., Mateos B., Iborra M., Nos P., Sandoval J., Beltrán B. Oxidative stress in the pathogenesis of Crohn’s disease and the interconnection with immunological response, microbiota, external environmental factors, and epigenetics. Antioxidants (Basel) 2021; 10(1): 64, https://doi.org/10.3390/antiox10010064.
- D’Haens G.R., Sartor R.B., Silverberg M.S., Petersson J., Rutgeerts P. Future directions in inflammatory bowel disease management. Journal of Crohn’s and Colitis 2014; 8: 726–734, https://doi.org/10.1016/j.crohns.2014.02.025.
- Martín Arranz E., Martín Arranz M.D., Robredo T., Mancheño-Corvo P., Menta R., Alves F.J., Suárez de Parga J.M., Mora Sanz P., de la Rosa O., Büscher D., Lombardo E., de Miguel F. Endoscopic submucosal injection of adipose-derived mesenchymal stem cells ameliorates TNBS-induced colitis in rats and prevents stenosis. Stem Cell Res Ther 2018; 9(1): 95, https://doi.org/10.1186/s13287-018-0837-x.
- Bernardi L., Santos C.H.M.D., Pinheiro V.A.Z., Oliveira R.J., Antoniolli-Silva A.C.M.B. Transplantation of adipose-derived mesenchymal stem cells in refractory Crohn’s disease: systematic review. Arq Bras Cir Dig 2019; 32(4): e1465, https://doi.org/10.1590/0102-672020190001e1465.
- Liu Y., Wang X., Hu C.A. Therapeutic potential of amino acids in inflammatory bowel disease. Nutrients 2017; 9(9): 920, https://doi.org/10.3390/nu9090920.
- da Costa Melo N.M., Almeida M.V.S., de Oliveira Campos D.M., de Oliveira C.B.S., Oliveira J.I.N. Animal models for inducing inflammatory bowel diseases: integrative review. REVISTA CIÊNCIAS EM SAÚDE 2021; 11(1): 80–87, https://doi.org/10.21876/rcshci.v11i1.1056.
- Low D., Nguyen D.D., Mizoguchi E. Animal models of ulcerative colitis and their application in drug research. Drug Des Devel Ther 2013; 7: 1341–1357, https://doi.org/10.2147/DDDT.S40107.
- Martin J.C., Bériou G., Josien R. Dextran sulfate sodium (DSS)-induced acute colitis in the rat. Methods Mol Biol 2016; 1371: 197–203, https://doi.org/10.1007/978-1-4939-3139-2_12.
- Waldner M.J., Neurath M.F. Chemically induced mouse models of colitis. Curr Protoc Pharmacol 2009; 1–15, https://doi.org/10.1002/0471141755.ph0555s46.
- Kolios G. Animal models of inflammatory bowel disease: how useful are they really? Curr Opin Gastroenterol 2016; 32(4): 251–257, https://doi.org/10.1097/MOG.0000000000000287.
- Golovenko O.V., Khomeriki S.G., Ivanova E.V., Fedorov E.D., Loranskaya I.D., Sitkin S.I., Belousova E.A., Golovenko A.O. Vospalitel’nye zabolevaniya kishechnika: klinicheskie, endoskopicheskie, morfologicheskie aspekty diagnostiki, printsipy sovremennoy terapii [Inflammatory bowel diseases: clinical, endoscopic, morphological aspects of diagnostics, principles of modern therapy]. Moscow: Prima Print; 2022; 258 p.
- Zheng J.J., Cu X.Q., Shi X.H., Wang Y.M., Jia L.M., Zhou X.L., Wang F.M. Colonoscopic and histologic features of colonic Crohn’s disease in Chinese patients. J Dig Dis 2007; 8(1): 35–41, https://doi.org/10.1111/j.1443-9573.2007.00281.x.










