Redox-Sensitive Fluorescent Nanoparticles for Biovisualization of Malignant Tumors
Application of fluorescent redox-sensitive nanoparticles in current biomedicine ensures high sensitivity and accuracy of biovisualization. Nanoparticles are potent as they can long circulate in the blood, where the level of glutathione is relatively low, and are destroyed in tumor cells, releasing loaded dyes or drugs.
The aim of the study was to develop new nanoparticles based on trithiocyanuric acid for biovisualization of malignant tumors and study capabilities of the developed nanoparticles.
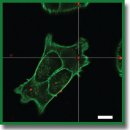
Materials and Methods. Nanoparticles were obtained by polycondensation of trithiocyanuric acid using iodine. Scanning and transmission electron microscopy was used for their characterization, the loading of fluorescent dyes was assessed by means of spectrophotometry. Confocal laser scanning microscopy was applied to study the impact of nanoparticles on the viability of the 4T1 and A549 cell lines as well as their interaction with cells. The distribution of nanoparticles in tissues and organs of BALB/c model mice with grafted tumors was performed using fluorescence visualization.
Results. According to scanning microscopy, the size of the synthesized particles reached 100±20 nm. The adsorption isotherm demonstrated that adsorption of 0.27 mg of the RhB fluorescent dye per 1 mg of nanoparticles could be achieved. Enhanced release of the packed fluorescent dye was seen in the presence of glutathione and acetylcysteine. The particles did not significantly affect the viability of 4T1 and A549 cells. After intratumoral administration, they ensured a more intense fluorescent signal in the tumor area compared to a regular fluorescent dye solution.
Conclusion. The developed system of trithiocyanuric-acid-based nanoparticles demonstrated high efficiency in biovisualization of malignant tumors and has a potential for targeted delivery of treatment agents.
- Wolfbeis O.S. An overview of nanoparticles commonly used in fluorescent bioimaging. Chem Soc Rev 2015; 44(14): 4743–4768, https://doi.org/10.1039/c4cs00392f.
- Li W., Kaminski Schierle G.S., Lei B., Liu Y., Kaminski C.F. Fluorescent nanoparticles for super-resolution imaging. Chem Rev 2022; 122(15): 12495–12543, https://doi.org/10.1021/acs.chemrev.2c00050.
- Lian W., Litherland S.A., Badrane H., Tan W., Wu D., Baker H.V., Gulig P.A., Lim D.V., Jin S. Ultrasensitive detection of biomolecules with fluorescent dye-doped nanoparticles. Anal Biochem 2004; 334(1): 135–144, https://doi.org/10.1016/j.ab.2004.08.005.
- Roy S., Bag N., Bardhan S., Hasan I., Guo B. Recent progress in NIR-II fluorescence imaging-guided drug delivery for cancer theranostics. Adv Drug Deliv Rev 2023; 197: 114821, https://doi.org/10.1016/j.addr.2023.114821.
- Al-Thani A.N., Jan A.G., Abbas M., Geetha M., Sadasivuni K.K. Nanoparticles in cancer theragnostic and drug delivery: a comprehensive review. Life Sci 2024; 352: 122899, https://doi.org/10.1016/j.lfs.2024.122899.
- Li Y., Chen Q., Pan X., Lu W., Zhang J. New insight into the application of fluorescence platforms in tumor diagnosis: from chemical basis to clinical application. Med Res Rev 2023; 43(3): 570–613, https://doi.org/10.1002/med.21932.
- Wang K., Du Y., Zhang Z., He K., Cheng Z., Yin L., Dong D., Li C., Li W., Hu Z., Zhang C., Hui H., Chi C., Tian J. Fluorescence image-guided tumour surgery. Nature Reviews Bioengineering 2023; 1(3): 161–179, https://doi.org/10.1038/s44222-022-00017-1.
- Shi Q., Xu J., Xu H., Wang Q., Huang S., Wang X., Wang P., Hu F. Polystyrene-based matrix to enhance the fluorescence of aggregation-induced emission luminogen for fluorescence-guided surgery. Small 2024; 20(22): e2309589, https://doi.org/10.1002/smll.202309589.
- Sutton P.A., van Dam M.A., Cahill R.A., Mieog S., Polom K., Vahrmeijer A.L., van der Vorst J. Fluorescence-guided surgery: comprehensive review. BJS Open 2023; 7(3): zrad049, https://doi.org/10.1093/bjsopen/zrad049.
- Bortot B., Mangogna A., Di Lorenzo G., Stabile G., Ricci G., Biffi S. Image-guided cancer surgery: a narrative review on imaging modalities and emerging nanotechnology strategies. J Nanobiotechnology 2023; 21(1): 155, https://doi.org/10.1186/s12951-023-01926-y.
- Ali M.K., Javaid S., Afzal H., Zafar I., Fayyaz K., Ain Q.U., Rather M.A., Hossain M.J., Rashid S., Khan K.A., Sharma R. Exploring the multifunctional roles of quantum dots for unlocking the future of biology and medicine. Environ Res 2023; 232: 116290, https://doi.org/10.1016/j.envres.2023.116290.
- Hang Y., Wang A., Wu N. Plasmonic silver and gold nanoparticles: shape- and structure-modulated plasmonic functionality for point-of-caring sensing, bio-imaging and medical therapy. Chem Soc Rev 2024; 53(6): 2932–2971, https://doi.org/10.1039/d3cs00793f.
- Farinha P., Coelho J.M.P., Reis C.P., Gaspar M.M. A comprehensive updated review on magnetic nanoparticles in diagnostics. Nanomaterials (Basel) 2021; 11(12): 3432, https://doi.org/10.3390/nano11123432.
- Ansari M.A., Shoaib S., Chauhan W., Gahtani R.M., Hani U., Alomary M.N., Alasiri G., Ahmed N., Jahan R., Yusuf N., Islam N. Nanozymes and carbon-dots based nanoplatforms for cancer imaging, diagnosis and therapeutics: current trends and challenges. Environ Res 2024; 241: 117522, https://doi.org/10.1016/j.envres.2023.117522.
- Hossain M.K., Khan M.I., El-Denglawey A. A review on biomedical applications, prospects, and challenges of rare earth oxides. Applied Materials Today 2021; 24: 101104, https://doi.org/10.1016/j.apmt.2021.101104.
- Ng K.K., Zheng G. Molecular interactions in organic nanoparticles for phototheranostic applications. Chem Rev 2015; 115(19): 11012–11042, https://doi.org/10.1021/acs.chemrev.5b00140.
- Chen M., Liu D., Liu F., Wu Y., Peng X., Song F. Recent advances of redox-responsive nanoplatforms for tumor theranostics. J Control Release 2021; 332: 269–284, https://doi.org/10.1016/j.jconrel.2021.02.030.
- Zhu C., Hu W., Wu H., Hu X. No evident dose-response relationship between cellular ROS level and its cytotoxicity — a paradoxical issue in ROS-based cancer therapy. Sci Rep 2014; 4: 5029, https://doi.org/10.1038/srep05029.
- Tobwala S., Fan W., Hines C.J., Folk W.R., Ercal N. Antioxidant potential of Sutherlandia frutescens and its protective effects against oxidative stress in various cell cultures. BMC Complement Altern Med 2014; 14: 271, https://doi.org/10.1186/1472-6882-14-271.










