Efficacy Evaluation of “Enhanced” Natural Killers with CISH and B2M Knockouts on Viability and Metabolic Status of 3D Glioblastoma Spheroid Cells in Patients
One of the alternative approaches to glioblastoma treatment is cellular immunotherapy based on natural killer cells (NK cells). To enhance their cytotoxic effect on tumor cells, new NK cell lines are being created using genetic engineering techniques.
The aim of the study was to evaluate the impact efficacy of “enhanced” NK cells on early metabolic rearrangements and the viability of glioblastoma cells in a patient using a tumor spheroid model.
Materials and Methods. The study used a primary culture of GBM7-Luc2-mKate2 human glioblastoma, a line of YT (YTwt) wild-type human NK cells, as well as lines created by us with overexpression of VAV1 protein with either CISH (YT–Vav1+CISH–/–) or B2M (YT–Vav1+B2M–/–) knockouts. Tumor spheroids were produced in round-bottomed, low-adhesive plates. 100 thousand immune cells were added to each spheroid, and spheroids viability was evaluated at several time points applying fluorescence staining using a live/dead cell viability assay kit; autofluorescence of metabolic coenzyme nicotinamide adenine dinucleotide (phosphate), or NAD(P)H, was visualized in spheroids using an LSM 880 laser scanning microscope (Carl Zeiss, Germany) with a FLIM module (Becker & Hickl GmbH, Germany).
Results. It was found that autofluorescence attenuation parameters of NAD(P)H coenzyme in human glioblastoma cells change significantly when exposed to both YT–Vav1+CISH–/– and YT–Vav1+B2M–/–, indicating occurrence of an early metabolic shift in tumor cells towards a less aggressive oxidative phenotype, and this is consistent with dead cells fraction increase and living cells fraction decrease in spheroid composition.
Conclusion. The data obtained on enhanced cytotoxic activity of new modified NK cell lines against human glioblastoma spheroids are important to understand interaction mechanisms between tumor and immune cells and the development of glioblastoma adoptive cell therapy.
Introduction
Among the existing approaches to malignant tumors treatment, one of the promising strategies is immunotherapy aimed at immune system activation against tumor cells [1, 2]. Immunotherapy has already proven its efficacy against a number of oncological diseases, and now scientists are working to develop personalized approaches and expand the range of its use localizations. Of particular interest is the development of glioblastoma immunotherapy, the most aggressive form of brain tumors. Until now, this remains a challenge as blood-brain barrier along with immunosuppressive tumor microenvironment hamper the achievement of a positive antitumor response [3, 4].
The use of natural killer cells (NK cells) is considered a promising area of cellular antitumor immunotherapy. These cells are a component of innate immunity and play a key role in recognizing and destroying cells infected with viruses and tumor cells. NK cells are functionally similar to CD8+ T lymphocytes, however, unlike them, they are able to recognize and act against pathological cells without additional sensitization by antigens [5, 6].
The cytotoxic activity of NK cells is controlled by inhibitory and activating receptors on their surface. As a rule, the cytotoxic activity of NK cells entering the tumor is low due to the influence of various immunosuppressive factors and regulatory T cells. To enhance the antitumor activity of NK cells, genetic engineering techniques are used, namely genetic constructs are introduced to increase the signaling proteins expression involved in cell activation and knockout of genes associated with their cytotoxicity inactivation [7–9]. It is assumed that the combination of such NK cell modifications can provide the most evident cytotoxic effect.
Earlier [10], we created two modified cell lines with increased cytotoxicity based on NK-like human YT cells with overexpression of VAV1 protein with CISH or B2M knockouts. VAV1 protein is a member of the family of guanine nucleotide exchange factors, one of the functions of which is the positive regulation of NK cell-mediated cytotoxicity [11]. The CISH gene encodes CIS, a cytokine-inducible SH2-containing protein. This gene produces a negative regulator of NK cells cytotoxicity, reducing their sensitivity to interleukin 15 (IL-15) [12]. The B2M gene encodes β2-microglobulin protein, a component of the light chain of the main histocompatibility complex class I (MHC I). B2M knockout disrupts MHC I NK cells structure making these cells invisible to host T lymphocytes, thereby opening up prospects for widespread clinical use of allogeneic NK cells [13].
Our previous study [10] demonstrated that modified NK cell lines eliminate primary glioblastoma cells in monolayer culture more efficiently than wild-type cells.
The aim of the study was to evaluate the efficacy of “enhanced” NK cells impact on early metabolic rearrangements and the viability of patients’ glioblastoma cells using a three-dimensional model of tumor spheroids.
Tumor spheroids are spherical multicellular aggregates with a heterogeneous structure and thus act as a more complex cellular model compared to standard monolayer cultures. Due to their spatial organization, spheroids allow to model tissue three-dimensional architecture with characteristic intercellular interactions and tight contacts, oxygen and nutrient gradients, variable levels of cells proliferative/metabolic activity and viability. Spheroids and similar multicellular structures (e.g. explants, organoids) obtained from the patients’ tumor materials are recognized as adequate models for preclinical testing and personalized selection of antitumor therapy [14].
Traditionally, cell viability and proliferative activity are the base to assess tumor cell response to therapy at in vitro systems using colorimetric methods, fluorescent dyes or immunocytochemistry. Fluorescence lifetime imaging microscopy (FLIM) of coenzyme NAD(P)H autofluorescence is considered a promising method to assess cellular response to therapy [15, 16]. This method allows to assess living cells metabolic status dynamics without additional staining and destruction considering intercellular heterogeneity. The “metabolic” FLIM is based on assessment of NAD(P)H fluorescence (attenuation) lifetime which varies for different coenzyme types — free and protein-bound, unphosphorylated, and phosphorylated. Metabolic perturbations accompany proliferation decrease or precede cell death after exposure and inevitably lead to changes of NAD(P)H fluorescence decay parameters [17, 18].
Thus, the therapeutic efficacy research of “enhanced” NK cells on patient-specific tumor spheroids with early response assessment using FLIM method is an innovative aspect of studying the described problem.
Materials and Methods
Cell cultures. A previously obtained primary culture of GBM7-Luc2-mKate2 patient’s glioblastoma was used to create tumor spheroids [19]. To obtain the required amount, tumor cells were cultured in DMEM nutrient medium (PanEco, Russia) according to the protocol we developed earlier [20].
The research was run using a line of NK-like wild-type human cells (YTwt) kindly provided by A.V. Filatov (Immunochemistry Laboratory of Moscow Institute of Immunology, Russia) along with modified lines created on this basis with overexpression of VAV1 protein with either CISH (YT–Vav1+CISH–/–) or B2M (YT–Vav1+B2M–/–) knockouts [10].
NK-like YT cell lines were cultured at the same conditions as tumor cells, but IL-2 (NPC Biotech, Russia) and IL-15 (Sino Biological, China) were always added to the nutrient medium (1 µl per 1 ml of medium). During passaging, the cells were centrifuged for 5 min at 300 g, and the precipitate was resuspended in a nutrient medium.
Tumor spheroids derivation. To obtain 3D tumor spheroids, 2000 cells along with 200 µl of nutrient medium were seeded to each well of 96-well low-adhesive round-bottomed plates (Corning, USA) [20]. Spheroid morphology was evaluated using a DM IL LED inverted microscope (Leica Microsystems, Germany).
On day 4 of growth, the spheroids were transferred using a pipette onto special plates with black walls and glass bottom (Gibco, USA) — 1 spheroid per well in DMEM nutrient medium without phenol red (Gibco, USA) followed by examination applying fluorescence microscopy.
100 thousand of YTwt, YT–Vav1+CISH–/– or YT–Vav1+B2M–/– cells were added to each spheroid according to a previously developed protocol [10].
Assessment of spheroids viability using fluorescent staining. To study the mechanisms of tumor cell death after incubation with NK cells, spheroids were stained using a live/dead cell viability assay kit (ab 176750; Abcam, Great Britain). According to the manufacturer’s protocol, a mixture of solutions was used — Nuclear Green DCS1 to stain necrotic/late apoptotic cells and CytoCalcein Violet 450 to stain viable cells. Fluorescent images of spheroids were obtained using a Leica DM IL LED inverted microscope (Leica Microsystems, Germany) with YFP (Ex: BP 500/20, Em: BP 535/30) filters for necrotic/late apoptotic cells and CFP (Ex: BP 436/20, Em: BP 480/40) filters for viable cells. The stained fraction of viable and necrotic/late apoptotic cells in the spheroid was calculated as a percentage using the ImageJ program (National Institutes of Health, USA).
Fluorescence microscopy and FLIM. An LSM 880 laser scanning confocal microscope (Carl Zeiss, Germany) was used during the research. The excitation source was a femtosecond Ti:Sa laser (Spectra Physics, USA) with 80 MHz repetition rate of 120 fs duration pulses and 690 to 1040 nm wavelength tunable range. The images were obtained using a 40×/1.3 oil immersion lens. During experiments, the spheroids were maintained in 5% CO2 medium at 37°C. To excite fluorescence of red mKate2 protein in spheroid tumor cells, 543 nm wavelength irradiation was applied, and a 650 nm wavelength signal was received.
Fluorescence lifetimes were detected using a FLIM module based on a time-correlated count of single TCSPC photons (Becker & Hickl, Germany). Coenzyme NAD(P)H fluorescence was called applying two photon 750 nm excitation, the received signal was within 450 to 490 nm wavelength range. The excitation irradiation power was 7 mW. The number of photons per pixel was not less than 5000 at ~60 s photon collection time.
To evaluate fluorescence decay parameters, SPCImage software (Becker & Hickl GmbH, Germany) was used. The decay was approximated applying the least squares method along with a bi-exponential function at 0.8 to 1.2 confidence interval of Chi-square approximation. The following decay parameters were calculated:  1 — the lifetime of the short component,
1 — the lifetime of the short component,  2 — the lifetime of the long component, α1 — the percentage contribution of the short component, α2 — the percentage contribution of the long component,
2 — the lifetime of the long component, α1 — the percentage contribution of the short component, α2 — the percentage contribution of the long component,  m — weighted mean lifetime (
m — weighted mean lifetime ( m=α1∙
m=α1∙ 1+α2∙
1+α2∙ 2)/(α1+α2). Short lifetime
2)/(α1+α2). Short lifetime  1 (0.3 to 0.5 ns) corresponds to free NAD(P)H produced during glycolysis process. Long lifetime
1 (0.3 to 0.5 ns) corresponds to free NAD(P)H produced during glycolysis process. Long lifetime  2 (1.2 to 2.5 ns) corresponds to protein-bound NAD(P)H, its contribution correlating with mitochondrial respiration activity.
2 (1.2 to 2.5 ns) corresponds to protein-bound NAD(P)H, its contribution correlating with mitochondrial respiration activity.
The cytoplasm area of each spheroid cell was examined. Images of 4 to 10 spheroids were obtained for each exposure and control with no exposure. 15 to 20 cells of each spheroid were examined.
Statistical analysis. GraphPad Prism 8.4.3 software (GraphPad Software, USA) was used for data comparative analysis and graphical representation. Data were checked for distribution normality using Shapiro–Wilk test (p≥0.05 distribution was considered normal). Since Shapiro–Wilk test revealed abnormal distribution, nonparametric Mann–Whitney U test was used to assess statistically significant differences between research groups, and p<0.05 differences were considered statistically significant.
Results
Assessment of human glioblastoma tumor spheroids viability after exposure to “enhanced” NK cells
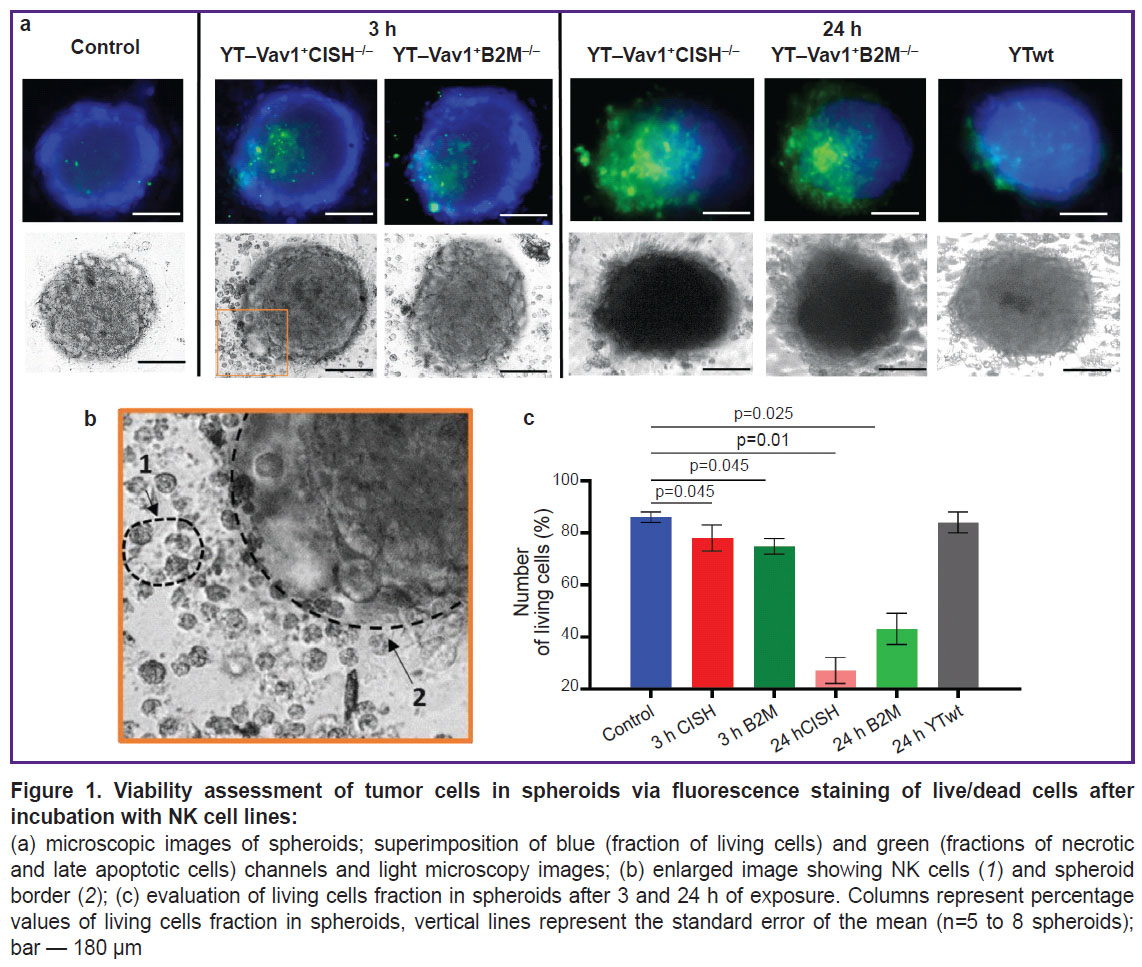
Cytotoxicity of “enhanced” NK cells against human glioblastoma tumor spheroids was assessed evaluating spheroid morphological state according to data of light microscopy and fluorescence staining using a live/dead cell viability assay kit 3 and 24 h after immune cells were added.
The control group spheroids (with no immune cells added) shape was spherical, their structure was dense with a clear and even border. They consisted mainly of living cell fraction (~86%). Necrotic cells accounted for ~14% and were observed only at the spheroid central zone (Figure 1).
Spheroid morphology showed no visible changes when spheroids where incubated with unmodified YTwt NK cell line for 24 h. Spheroids retained their dense structure, and a small number of detached cells was observed at spheroid periphery. No difference in viable cell fraction was found compared to the intact control (~84%).
After 3 h of incubation with modified YT–Vav1+CISH–/– and YT–Vav1+B2M–/– NK cell lines, the spheroids lost their compact structure and tumor cells detached along the edge. Fluorescence microscopy showed that spheroid dead cell fraction increased (~21% for YT–Vav1+CISH–/–, ~25% for YT–Vav1+B2M–/–) and, accordingly, living cell fraction decreased (~78% for YT–Vav1+CISH–/–, ~74% for YT–Vav1+B2M–/–; p=0.045 compared with the control). At light microscopy images, a tumor spheroid surrounds an NK cell population with some cells at spheroid periphery. NK cells morphology was typical — rounded cells with evident granular cytoplasm.
24 h after exposure was started, the spheroids showed signs of destruction. At spheroid border, evident detachment of cells at the points of contact with immune cells was observed, the spheroid became more elongated and less dense due to intercellular contacts loss. The fraction of dead tumor cells increased to ~71% for YT–Vav1+CISH–/– and to ~58% for YT–Vav1+B2M–/– (p=0.01 and p=0.025, respectively, compared to the control with no exposure).
Thus, the viability assessment of human glioblastoma spheroids after exposure to modified NK cell lines demonstrated enhanced cytotoxicity of YT-Vav1+CISH–/– and YT–Vav1+B2M–/– cells compared with standard unmodified YTwt line, this being consistent with earlier experiments on a human glioblastoma monolayer culture of [10].
Assessment of tumor spheroid cell metabolic status after exposure to “enhanced” NK cells using FLIM microscopy
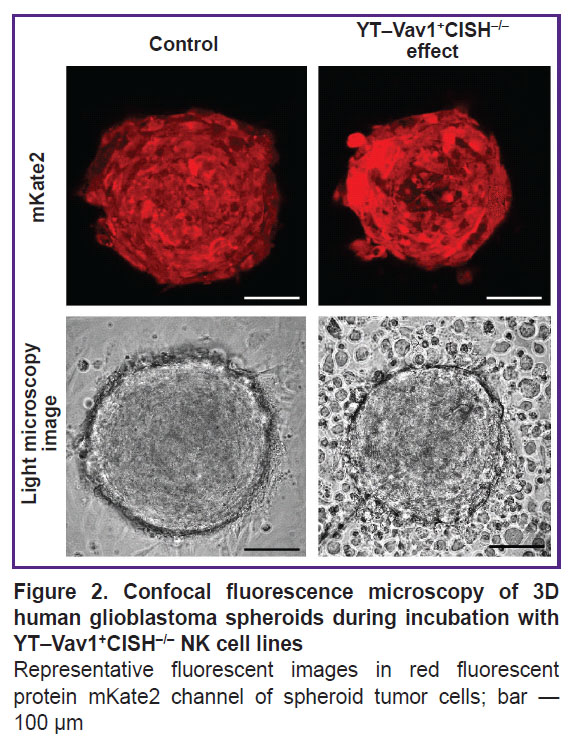
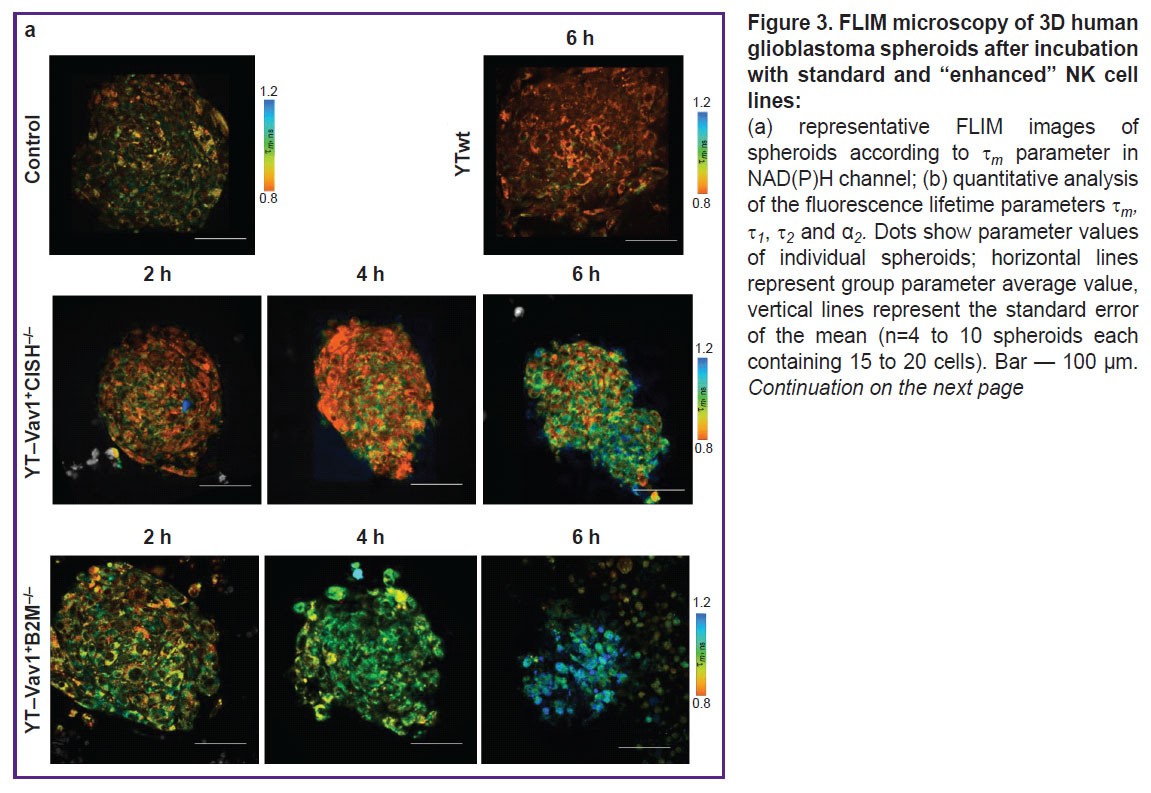
FLIM microscopy was used to obtain images of NAD(P)H coenzyme autofluorescence in spheroid cells of patient’s glioblastoma and to assess lifetime parameters. To study the effect of NK cells on tumor cell metabolism, it was assessed 6 h after incubation with wild-type YTwt line and 2, 4, and 6 h after YT–Vav1+CISH–/– or YT–Vav1+B2M–/– knockout lines were added. To identify spheroid tumor cells, fluorescent confocal images of spheroids were obtained without and with addition of NK cell lines to the fluorescence channel of red protein mKate2 (Figure 2).
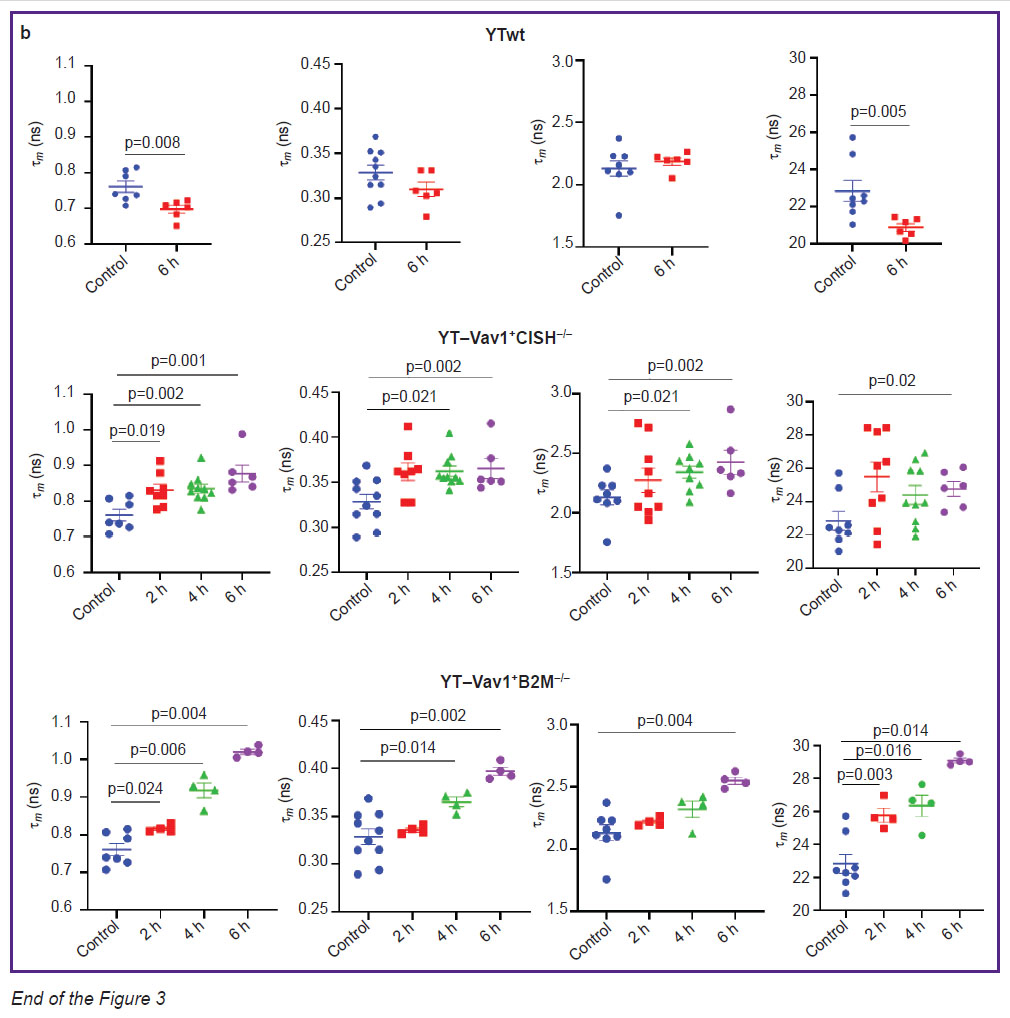
Effects of YTwt cells on tumor spheroids. In the control (with spheroid no exposure to immune cells), typical NAD(P)H fluorescence lifetime values were noted:  1 ~0.33 ns for short component,
1 ~0.33 ns for short component,  2 ~2.1 ns for long component, ~77 and 22% short and long component contribution α1 and α2, respectively, ~0.76 ns
2 ~2.1 ns for long component, ~77 and 22% short and long component contribution α1 and α2, respectively, ~0.76 ns  m average lifetime. After 6 h of incubation with unmodified YTwt NK cells, a statistically significant decrease to 0.7±0.01 ns (p=0.008) was observed of
m average lifetime. After 6 h of incubation with unmodified YTwt NK cells, a statistically significant decrease to 0.7±0.01 ns (p=0.008) was observed of  m parameter resulting from decrease to 20.9±0.2% (p=0.005) of α2 bound NAD(P)H relative contribution (Figure 3). Reduced bound NAD(P)H contribution may indicate tumor cell increased glycolysis, in its turn associated with retention and enhancement of glioblastoma cells aggressive phenotype despite the therapeutic effect.
m parameter resulting from decrease to 20.9±0.2% (p=0.005) of α2 bound NAD(P)H relative contribution (Figure 3). Reduced bound NAD(P)H contribution may indicate tumor cell increased glycolysis, in its turn associated with retention and enhancement of glioblastoma cells aggressive phenotype despite the therapeutic effect.
Effects of YT–Vav1+CISH–/– and YT–Vav1+B2M–/– NK cells on tumor spheroids. Evident changes in all lifetime parameters were detected in tumor spheroids exposed to YT–Vav1+CISH–/– knockout NK cells. After 2 h of incubation, the values of parameters increased:  1 from 0.33±0.01 to 0.36±0.01 ns (p=0.019);
1 from 0.33±0.01 to 0.36±0.01 ns (p=0.019);  2 from 2.1±0.6 to 2.2±0.9 ns; α2 from 22.8±0.10 to 25.9±0.9%;
2 from 2.1±0.6 to 2.2±0.9 ns; α2 from 22.8±0.10 to 25.9±0.9%;  m from 0.76±0.02 to 0.83±0.20 ns (p=0.019). At the 4th hour of incubation,
m from 0.76±0.02 to 0.83±0.20 ns (p=0.019). At the 4th hour of incubation,  1 and
1 and  m values remained at the same level, changes of
m values remained at the same level, changes of  2 became statistically significant (2.3±0.5 ns; p=0.021). At the 6th hour, increase was observed:
2 became statistically significant (2.3±0.5 ns; p=0.021). At the 6th hour, increase was observed:  1 to 0.37±0.10 ns (p=0.017),
1 to 0.37±0.10 ns (p=0.017),  2 to 2.5±0.9 ns (p=0.02), α2 to 24.2±0.5%, this resulting in
2 to 2.5±0.9 ns (p=0.02), α2 to 24.2±0.5%, this resulting in  m average lifetime increase to 0.88±0.20 s (p=0.001 compared to control with no exposure).
m average lifetime increase to 0.88±0.20 s (p=0.001 compared to control with no exposure).
Incubation of human glioblastoma spheroids with YT-Vav1+B2M–/– knockout NK cells also showed a 6-hour gradual increase of all fluorescence lifetime parameters. After 2 h of incubation, significant changes occur of α2 parameter with statistically significant increase of values from 22.8±0.1% to 25.8±0.4% (p=0.03), this resulting in  m average lifetime increase from 0.76±0.02 to 0.82±0.04 ns (p=0.024). At the 4th hour of exposure, a statistically significant increase of
m average lifetime increase from 0.76±0.02 to 0.82±0.04 ns (p=0.024). At the 4th hour of exposure, a statistically significant increase of  1 was detected — from 0.33±0.01 to 0.36±0.03 ns (p=0.014) compared to control spheroids. After 6 h of spheroid incubation with immune cells, all lifetime parameters showed higher values compared to intact control; higher 1.02±0.06 ns (p=0.004)
1 was detected — from 0.33±0.01 to 0.36±0.03 ns (p=0.014) compared to control spheroids. After 6 h of spheroid incubation with immune cells, all lifetime parameters showed higher values compared to intact control; higher 1.02±0.06 ns (p=0.004)  m values reflected this. It should be noted that effect of YT–Vav1+B2M–/– cells caused lower spread of values within spheroid groups for each time point compared to effect of YT–Vav1+CISH–/– cells; this indicates greater reproducibility and stability of metabolic dysfunction.
m values reflected this. It should be noted that effect of YT–Vav1+B2M–/– cells caused lower spread of values within spheroid groups for each time point compared to effect of YT–Vav1+CISH–/– cells; this indicates greater reproducibility and stability of metabolic dysfunction.
Thus, the effect of modified NK cells on human glioblastoma spheroids has evident cytotoxic impact combined with metabolic rearrangements at cellular level that could be detected applying FLIM method to NAD(P)H coenzyme.
Discussion
Immunotherapy demonstrates high potential as a treatment method of glioblastoma. Adoptive cell therapy is actively developed — immune cells are obtained from a patient, cultured in vitro to increase their number, further modified, and are injected into the body to fight the tumor. NK cells look promising as an antitumor agent for adoptive cell therapy due to their ability to recognize and destroy malignant cells without requiring antigen-specific activation. However, despite their high therapeutic potential, native NK cells efficacy is low due to the immunosuppressive microenvironment of tumors; therefore, methods are currently being actively developed to “enhance” NK cells cytotoxicity and overcome the mechanisms of immune surveillance evasion. Various cell modification techniques are used for this purpose, e.g. creation of chimeric antigenic receptors or specific knockout genes responsible for reducing NK cells cytotoxicity [21]. Currently, preclinical studies have demonstrated the high efficacy of NK therapy using “enhanced” cells for various types of oncological diseases, including leukemia, lymphoma, myeloma, ovarian cancer [22]. At the same time, treatment of solid tumors using NK cells, in particular glioblastoma [23], causes certain difficulties; therefore, creation of new effective NK cell lines and studies of their cytotoxicity remain urgent research areas.
The most widely used cytokine for NK cell therapy in clinical trials is IL-15. Besides, IL-15 receptor is expressed on both NK cells and CD8+ T cells, but, importantly, not on regulatory T cells [24]. The latter provides a key advantage of their use compared to IL-2-based therapy [25]. Increasing number of publications evidences that exogenous administration of IL-15 prevents loss of NK cell effector functions in tumor microenvironment.
However, continuous treatment using human NK cells via IL-15 can cause depletion of these cells, so both the treatment regimen and the administration method to achieve maximum benefit for patients should be carefully considered [26].
Previously [10], we were able to obtain CISH knockout lines allowing to increase NK cells cytotoxicity without adding exogenous IL-15 to the culture. Experiments showed that NK cells with CISH knockout had a more effective impact on the monolayer of human primary glioblastoma culture.
In addition, the method of metabolic fluorescent time-resolved imaging allowed to obtain valuable data on tumor metabolism features from the model of human glioblastoma 3D spheroids [20]. Combined results allowed to evaluate the efficacy of YT-Vav1+CISH–/– and YT–Vav1+B2M–/– modified NK cell lines at conditions close to tumor process development in the human body.
In this research, antitumor activity of YT-Vav1+CISH–/– and YT–Vav1+B2M–/– modified NK cell lines was evaluated using a model of 3D tumor spheroids of a patient’s glioblastoma. The focus of the research was on the study of early metabolic rearrangements in tumor cells during NK therapy using the FLIM optical method. At the moment, metabolic aspects of NK therapy are poorly understood. The advantage of FLIM is the possibility to perform the dynamic observations of living cells with (sub)cellular resolution compared to biochemical, immunocytochemical, and molecular genetic methods of cellular metabolic status assessment.
It was found that all autofluorescence decay parameters of NAD(P)H coenzyme in human glioblastoma cells significantly change when exposed to YT-Vav1+CISH–/– and YT–Vav1+B2M–/– modified NK cells; this indicates that tumor cell metabolic status is changed and is consistent with data on spheroid dead cells fraction increase and living cells fraction decrease and, accordingly, on effective response to therapeutic effects. In a typical case, contribution growth of bound α2 NAD(P)H in cells is associated with increased mitochondrial respiration. In one of our studies [20] using FLIM method, we showed that relative contributions of α1 and α2 NAD(P)H adequately represent metabolic rearrangements in spheroids of primary glioblastoma in patients as response to oxygen content decrease in their microenvironment. During research [18], we found that α2 NAD(P)H is strongly correlated to survival as well as to proliferative activity of tumor cells isolated from gliomas of various patients treated with temozolomide. α2 NAD(P)H contribution increase in tumor cells as a result of switching to a more oxidative metabolism is a typical reaction to various types of cytotoxic chemotherapy shown in vitro and in vivo at numerous researches of our group and of other laboratories [27–31].
In addition to changes of α2 bound NAD(P)H relative contribution, NK cells effects on tumor cells also caused absolute lifetime values increase of  1 free and
1 free and  2 protein-bound NAD(P)H fluorescence. A number of factors like pH, temperature, and viscosity of local microenvironment affect fluorescence lifetime of various fluorophores, but these factors influence within physiological values range is insignificant for NAD(P)H. A recent study of Song et al. [32] showed that
2 protein-bound NAD(P)H fluorescence. A number of factors like pH, temperature, and viscosity of local microenvironment affect fluorescence lifetime of various fluorophores, but these factors influence within physiological values range is insignificant for NAD(P)H. A recent study of Song et al. [32] showed that  1 free NAD(P)H lifetime depends on the size of oxidized (NAD+) total pool and reduced
1 free NAD(P)H lifetime depends on the size of oxidized (NAD+) total pool and reduced  1 coenzyme (NADH) increases with pool decrease and decreases with pool increase. Lifetime of
1 coenzyme (NADH) increases with pool decrease and decreases with pool increase. Lifetime of  2 enzyme-bound NAD(P)H depends on specific enzyme availability in a particular metabolic pathway [33].
2 enzyme-bound NAD(P)H depends on specific enzyme availability in a particular metabolic pathway [33].
Activity of many key enzymes of glucose metabolic pathways, including NAD(P)H-binding proteins, is altered during glioblastoma development [34]. Recent researches using FLIM method indicate a relationship of  2 NAD(P)H to mitochondrial cell activity. Morrow et al. [35] demonstrated that
2 NAD(P)H to mitochondrial cell activity. Morrow et al. [35] demonstrated that  2 NAD(P)H increases significantly during cell aging. Sanchez et al. research [36] found evident decrease of
2 NAD(P)H increases significantly during cell aging. Sanchez et al. research [36] found evident decrease of  1 and
1 and  2 in severe mitochondrial dysfunction. It was shown that increased mitochondrial activity is one of the signs of tumor oxidative phenotype. This suggests that
2 in severe mitochondrial dysfunction. It was shown that increased mitochondrial activity is one of the signs of tumor oxidative phenotype. This suggests that  2 NAD(P)H growth when “enhanced” NK cells produce their effect is also associated with a metabolic shift towards oxidative phosphorylation. Obtained data are consistent with our previous research [18] on temozolomide effect against patient’s glioblastoma cells, where we also observed an increase of
2 NAD(P)H growth when “enhanced” NK cells produce their effect is also associated with a metabolic shift towards oxidative phosphorylation. Obtained data are consistent with our previous research [18] on temozolomide effect against patient’s glioblastoma cells, where we also observed an increase of  1 and
1 and  2 NAD(P)H at therapeutic effect.
2 NAD(P)H at therapeutic effect.
Metabolic reprogramming, namely glycolysis activation, is an important biological feature of glioblastoma. Active aerobic glycolysis is used not only for accelerated production of ATP molecules, but also for metabolic intermediates synthesis necessary to maintain rapid growth and proliferation of glioblastoma. Reprogramming of cellular metabolism is closely related to changes of signaling pathways, in particular PI3K-Akt-mTOR; its activation in glioblastoma cells enhances glucose molecules absorption by cells and alters the activity of certain enzymes involved in glycolysis to meet the cell increased energy needs [33]. The shift of tumor cell metabolic balance towards oxidative phosphorylation as a result of treatment is considered a favorable factor and is associated with transition to a less aggressive phenotype. With tumor oxidative status, increased oxygen consumption making the tumor more sensitive to hypoxia is observed along with decreased lactate production making tumor microenvironment more favorable for attack of effector T cells and natural killers, increased mitochondrial activity, nucleotide and lipid biosynthesis weakening, and decrease of tumor cells proliferative activity [37].
It should be noted that the YTwt wild-type NK cell line demonstrates no evident cytotoxic effect on glioblastoma cells unlike modified lines. Besides, FLIM data revealed α2 NAD(P)H value decrease, most likely associated with increased glycolysis and a more aggressive phenotype. One of the possible reasons of unmodified NK cells low cytotoxic activity established in our research may be the short co-culture time. So, the research of Heinrich et al. [38] showed the destruction of spheroids of the human glioblastoma U87MG line only after 5 days or more of incubation with NK cells.
Thus, the new YT–Vav1+CISH–/– and YT–Vav1+B2M–/– modified NK cell lines demonstrate greater cytotoxic activity against primary patient’s glioblastoma spheroids compared with the standard line, causing early metabolic rearrangements and evident decrease of tumor cells viability.
Conclusion
Efficacy evaluation of new Vav1+CISH-/– and YT–Vav1+B2M–/– modified NK cell lines on a 3D glioblastoma tumor spheroids model via assessment of glioblastoma cells viability using fluorescent staining and of their metabolic status using the innovative FLIM method demonstrated the high cytotoxic activity of “enhanced” NK cell lines compared to the unmodified standard line. Early (after 2 h) metabolic rearrangements preceding cell death were shown of glioblastoma cells towards oxidative status caused by new modified lines effects. The obtained results are important to understand interaction mechanisms between glioblastoma and NK cells and the development of glioblastoma adoptive cell therapy.
Research funding. The research was supported by the Russian Science Foundation grant (No.22-64-00057).
Conflicts of interests. The authors have no conflicts of interest to declare.
References
- Lim M., Xia Y., Bettegowda C., Weller M. Current state of immunotherapy for glioblastoma. Nat Rev Clin Oncol 2018; 15(7): 422–442, https://doi.org/10.1038/s41571-018-0003-5.
- Mahmoud A.B., Ajina R., Aref S., Darwish M., Alsayb M., Taher M., AlSharif S.A., Hashem A.M., Alkayyal A.A. Advances in immunotherapy for glioblastoma multiforme. Front Immunol 2022; 13: 944452, https://doi.org/10.3389/fimmu.2022.944452.
- Liu Y., Zhou F., Ali H., Lathia J.D., Chen P. Immunotherapy for glioblastoma: current state, challenges, and future perspectives. Cell Mol Immunol 2024; 21(12): 1354–1375, https://doi.org/10.1038/s41423-024-01226-x.
- Mamessier E., Sylvain A., Thibult M.L., Houvenaeghel G., Jacquemier J., Castellano R., Gonçalves A., André P., Romagné F., Thibault G., Viens P., Birnbaum D., Bertucci F., Moretta A., Olive D. Human breast cancer cells enhance self tolerance by promoting evasion from NK cell antitumor immunity. J Clin Invest 2011; 121(9): 3609–3622, https://doi.org/10.1172/JCI45816.
- Suen W.C., Lee W.Y., Leung K.T., Pan X.H., Li G. Natural killer cell-based cancer immunotherapy: a review on 10 years completed clinical trials. Cancer Invest 2018; 36(8): 431–457, https://doi.org/10.1080/07357907.2018.1515315.
- Cooksey L.C., Friesen D.C., Mangan E.D., Mathew P.A. Prospective molecular targets for natural killer cell immunotherapy against glioblastoma multiforme. Cells 2024; 13(18): 1567, https://doi.org/10.3390/cells13181567.
- Bryceson Y.T., Chiang S.C., Darmanin S., Fauriat C., Schlums H., Theorell J., Wood S.M. Molecular mechanisms of natural killer cell activation. J Innate Immun 2011; 3(3): 216–226, https://doi.org/10.1159/000325265.
- da Silva L.H.R., Catharino L.C.C., da Silva V.J., Evangelista G.C.M., Barbuto J.A.M. The war is on: the immune system against glioblastoma-how can NK cells drive this battle? Biomedicines 2022; 10(2): 400, https://doi.org/10.3390/biomedicines10020400.
- Malmberg K.J., Carlsten M., Björklund A., Sohlberg E., Bryceson Y.T., Ljunggren H.G. Natural killer cell-mediated immunosurveillance of human cancer. Semin Immunol 2017; 31: 20–29, https://doi.org/10.1016/j.smim.2017.08.002.
- Yusubalieva G.M., Dashinimaev E.B., Gorchakov A.A., Kulemzin S.V., Brovkina O.A., Kalinkin A.A., Vinokurov A.G., Shirmanova M.V., Baklaushev V.P. Enhanced natural killers with CISH and B2M gene knockouts reveal increased cytotoxicity in glioblastoma primary cultures. Mol Biol 2022; 56: 770–779, https://doi.org/10.1134/S0026893322050156.
- Chan G., Hanke T., Fischer K.D. Vav-1 regulates NK T cell development and NK cell cytotoxicity. Eur J Immunol 2001; 31(8): 2403–2410, https://doi.org/10.1002/1521-4141(200108)31:8<2403::aid-immu2403>3.0.co;2-o.
- Delconte R.B., Kolesnik T.B., Dagley L.F., Rautela J., Shi W., Putz E.M., Stannard K., Zhang J.G., Teh C., Firth M., Ushiki T., Andoniou C.E., Degli-Esposti M.A., Sharp P.P., Sanvitale C.E., Infusini G., Liau N.P., Linossi E.M., Burns C.J., Carotta S., Gray D.H., Seillet C., Hutchinson D.S., Belz G.T., Webb A.I., Alexander W.S., Li S.S., Bullock A.N., Babon J.J., Smyth M.J., Nicholson S.E., Huntington N.D. CIS is a potent checkpoint in NK cell-mediated tumor immunity. Nat Immunol 2016; 17(7): 816–824, https://doi.org/10.1038/ni.3470.
- Höglund P., Glas R., Ménard C., Kåse A., Johansson M.H., Franksson L., Lemmonier F., Kärre K. Beta2-microglobulin-deficient NK cells show increased sensitivity to MHC class I-mediated inhibition, but self tolerance does not depend upon target cell expression of H-2Kb and Db heavy chains. Eur J Immunol 1998; 28(1): 370–378, https://doi.org/10.1002/(SICI)1521-4141(199801)28:01%3C370::AID-IMMU370%3E3.0.CO;2-W.
- Mitrakas A.G., Tsolou A., Didaskalou S., Karkaletsou L., Efstathiou C., Eftalitsidis E., Marmanis K., Koffa M. Applications and advances of multicellular tumor spheroids: challenges in their development and analysis. Int J Mol Sci 2023; 24(8): 6949, https://doi.org/10.3390/ijms24086949.
- Shcheslavsky V.I., Shirmanova M.V., Eltsov A., Becker V. Luminescence microscopy based on multiparameter time-correlated photon counting. Uspekhi biologicheskoy khimii 2019; 59: 103–138.
- Multimodal optical diagnostics of cancer. Tuchin V.V., Popp J., Zakharov V. (editors). Cham: Springer International Publishing; 2020.
- Druzhkova I., Komarova A., Nikonova E., Baigildin V., Mozherov A., Shakirova Y., Lisitsa U., Shcheslavskiy V., Ignatova N., Shirshin E., Shirmanova M., Tunik S. Monitoring the intracellular pH and metabolic state of cancer cells in response to chemotherapy using a combination of phosphorescence lifetime imaging microscopy and fluorescence lifetime imaging microscopy. Int J Mol Sci 2023; 25(1): 49, https://doi.org/10.3390/ijms25010049.
- Yuzhakova D.V., Sachkova D.A., Shirmanova M.V., Mozherov A.M., Izosimova A.V., Zolotova A.S., Yashin K.S. Measurement of patient-derived glioblastoma cell response to temozolomide using fluorescence lifetime imaging of NAD(P)H. Pharmaceuticals (Basel) 2023; 16(6): 796, https://doi.org/10.3390/ph16060796.
- Yuzhakova D., Kiseleva E., Shirmanova M., Shcheslavskiy V., Sachkova D., Snopova L., Bederina E., Lukina M., Dudenkova V., Yusubalieva G., Belovezhets T., Matvienko D., Baklaushev V. Highly invasive fluorescent/bioluminescent patient-derived orthotopic model of glioblastoma in mice. Front Oncol 2022; 12: 897839, https://doi.org/10.3389/fonc.2022.897839.
- Yuzhakova D.V., Lukina M.M., Sachkova D.A., Yusubalieva G.M., Baklaushev V.P., Mozherov A.M., Dudenkova V.V., Gavrina A.I., Yashin K.S., Shirmanova M.V. Development of a 3D tumor spheroid model from the patient’s glioblastoma cells and its study by metabolic fluorescence lifetime imaging. Sovremennye tehnologii v medicine 2023; 15(2): 28–38, https://doi.org/10.17691/stm2023.15.2.03.
- Tao J.H., Zhang J., Li H.S., Zhou Y., Guan C.X. Nature killer cell for solid tumors: current obstacles and prospective remedies in NK cell therapy and beyond. Crit Rev Oncol Hematol 2025; 205: 104553, https://doi.org/10.1016/j.critrevonc.2024.104553.
- Chu J., Gao F., Yan M., Zhao S., Yan Z., Shi B., Liu Y. Natural killer cells: a promising immunotherapy for cancer. J Transl Med 2022; 20(1): 240, https://doi.org/10.1186/s12967-022-03437-0.
- Feldman L., Brown C., Badie B. Chimeric antigen receptor (CAR) T cell therapy for glioblastoma. Neuromolecular Med 2022; 24(1): 35–40, https://doi.org/10.1007/s12017-021-08689-5.
- Rautela J., Huntington N.D. IL-15 signaling in NK cell cancer immunotherapy. Curr Opin Immunol 2017; 44: 1–6, https://doi.org/10.1016/j.coi.2016.10.004.
- Ahmadzadeh M., Rosenberg S.A. IL-2 administration increases CD4+ CD25(hi) Foxp3+ regulatory T cells in cancer patients. Blood 2006; 107(6): 2409–2414, https://doi.org/10.1182/blood-2005-06-2399.
- Felices M., Lenvik A.J., McElmurry R., Chu S., Hinderlie P., Bendzick L., Geller M.A., Tolar J., Blazar B.R., Miller J.S. Continuous treatment with IL-15 exhausts human NK cells via a metabolic defect. JCI Insight 2018; 3(3): e96219, https://doi.org/10.1172/jci.insight.96219.
- Lukina M.M., Dudenkova V.V., Shimolina L.E., Snopova L.B., Zagaynova E.V., Shirmanova M.V. In vivo metabolic and SHG imaging for monitoring of tumor response to chemotherapy. Cytometry A 2019; 95(1): 47–55, https://doi.org/10.1002/cyto.a.23607.
- Lukina M.M., Dudenkova V.V., Ignatova N.I., Druzhkova I.N., Shimolina L.E., Zagaynova E.V., Shirmanova M.V. Metabolic cofactors NAD(P)H and FAD as potential indicators of cancer cell response to chemotherapy with paclitaxel. Biochim Biophys Acta Gen Subj 2018; 1862(8): 1693–1700, https://doi.org/10.1016/j.bbagen.2018.04.021.
- Shirmanova M.V., Druzhkova I.N., Lukina M.M., Dudenkova V.V., Ignatova N.I., Snopova L.B., Shcheslavskiy V.I., Belousov V.V., Zagaynova E.V. Chemotherapy with cisplatin: insights into intracellular pH and metabolic landscape of cancer cells in vitro and in vivo. Sci Rep 2017; 7(1): 8911, https://doi.org/10.1038/s41598-017-09426-4.
- Shah A.T., Demory Beckler M., Walsh A.J., Jones W.P., Pohlmann P.R., Skala M.C. Optical metabolic imaging of treatment response in human head and neck squamous cell carcinoma. PLoS One 2014; 9(3): e90746, https://doi.org/10.1371/journal.pone.0090746.
- Alam S.R., Wallrabe H., Svindrych Z., Chaudhary A.K., Christopher K.G., Chandra D., Periasamy A. Investigation of mitochondrial metabolic response to doxorubicin in prostate cancer cells: an NADH, FAD and tryptophan FLIM assay. Sci Rep 2017; 7(1): 10451, https://doi.org/10.1038/s41598-017-10856-3.
- Song A., Zhao N., Hilpert D.C., Perry C., Baur J.A., Wallace D.C., Schaefer P.M. Visualizing subcellular changes in the NAD(H) pool size versus redox state using fluorescence lifetime imaging microscopy of NADH. Commun Biol 2024; 7(1): 428, https://doi.org/10.1038/s42003-024-06123-7.
- Zhao J., Ma X., Gao P., Han X., Zhao P., Xie F., Liu M. Advancing glioblastoma treatment by targeting metabolism. Neoplasia 2024; 51: 100985, https://doi.org/10.1038/s42003-024-06123-7.
- Principles of fluorescence spectroscopy. Lakowicz J.R. (editor). Boston: Springer US; 2006.
- Morrow C.S., Yao P., Vergani-Junior C.A., Anekal P.V., Llopis P.M., Miller J.W., Benayoun B.A., Mair W.B. Endogenous mitochondrial NAD(P)H fluorescence can predict lifespan. Commun Biol 2024; 7: 1551, https://doi.org/10.1038/s42003-024-07243-w.
- Sanchez T., Wang T., Pedro M.V., Zhang M., Esencan E., Sakkas D., Needleman D., Seli E. Metabolic imaging with the use of fluorescence lifetime imaging microscopy (FLIM) accurately detects mitochondrial dysfunction in mouse oocytes. Fertil Steril 2018; 110(7): 1387–1397, https://doi.org/10.1016/j.fertnstert.2018.07.022.
- Yang J., Shay C., Saba N.F., Teng Y. Cancer metabolism and carcinogenesis. Exp Hematol Oncol 2024; 13(1): 10, https://doi.org/10.1186/s40164-024-00482-x.
- Heinrich M.A., Huynh N.T., Heinrich L., Prakash J. Understanding glioblastoma stromal barriers against NK cell attack using tri-culture 3D spheroid model. Heliyon 2024; 10(3): e24808, https://doi.org/10.1016/j.heliyon.2024.e24808.